The 2010 Lasker~DeBakey Clinical Medical Research Award honors a scientist who discovered vascular endothelial growth factor (VEGF), a key participant in blood-vessel formation, and exploited this knowledge to devise an effective treatment for wet age-related macular degeneration (AMD).
Napoleone Ferrara (Genentech) has provided a therapy that can, for the first time, improve sight for people with this illness, many of whom were previously destined for blindness. Ferrara’s work has uncovered the lynchpin for one of the body’s most important physiological processes and unlocked a novel approach for combating a serious eye disorder.
People have known since Aristotle’s time that blood nourishes the body, yet the molecular underpinnings of blood-vessel formation remained mysterious for millennia. Initial insights arose from the study of cancer and eye disorders. Starting in the late 19th century, scientists reported that blood vessels proliferate before and during tumor expansion and that rapid tumor growth depends on a rich vascular supply. They documented that tumor cells release diffusible factors that foster angiogenesis, the creation and remodeling of new blood vessels from existing ones. The eye, too, could host troublesome blood-vessel growth, and in 1948, Isaac C. Michaelson (University of Glasgow) proposed that the retina contains an angiogenic factor that contributes to a form of blindness associated with premature birth.
These ideas found their first theoretical clinical application in 1971, when Judah Folkman (Harvard Medical School) suggested that restraining angiogenesis could thwart cancer. Although this concept sparked tremendous research activity, no one gained much traction in identifying physiologically relevant angiogenic factors — or in finding ways to block them — until Ferrara’s breakthrough.
In the 1980s, many groups reported proteins that promoted growth of vascular cells. Available technology and information, however, did not permit easy identification of the genes responsible for their production, which was essential to confirm the molecules’ biological roles. By the mid 1980s, two proteins — acidic and basic fibroblast growth factor (aFGF and bFGF, respectively) — had become frontrunner candidates. They spurred blood vessel cells as well as a wide range of other cells to divide in culture and induced angiogenesis when placed at sites in an animal that normally lack blood vessels. However, analysis of aFGF and bFGF genes revealed a snag: They did not contain the hallmark sequence that allows proteins to exit cells. It was hard to explain how an intracellular molecule could diffuse and prompt cells in neighboring blood vessels to reproduce and migrate.
When Ferrara began a postdoctoral fellowship at the University of California, San Francisco (UCSF) in the early 1980s, he was studying particular cells in the cow pituitary gland that seemed likely to help regulate blood-vessel development. Ferrara cultivated these cells in laboratory dishes and collected the broth in which they grew. When he added this medium to cells that line blood vessels — vascular endothelial cells — they divided vigorously. In contrast, cells from other tissues ignored the material. The active substance thus fulfilled key requirements for being a secreted molecule that provokes growth of vascular endothelial cells. Ferrara pursued the protein after he moved to Genentech in 1988. The following year, he isolated the gene that encodes it from cows as well as humans, and discovered that its amino acid sequence did not match that of any known protein. Crucially, it contained a sequence that allows proteins to escape from cells. Because the molecule exerts its effect only on vascular endothelial cells, Ferrara called it vascular endothelial growth factor (VEGF).
Simultaneously, an independent group at Monsanto Company, led by Daniel Connolly, uncovered the gene for vascular permeability factor (VPF), a protein from guinea pig tumors that Harold Dvorak (Harvard Medical School) had identified in 1983 on the basis of its ability to incite blood-vessel leakage. Sequence comparison showed that the VPF and VEGF genes were identical.
Thirsty for blood
VEGF could induce proliferation of blood-vessel cells in culture, but no one knew whether it did so in animals. Ferrara showed that, in rats, Vegf gene activity correlates temporally with blood-vessel growth and VEGF binding correlates spatially with blood vessels. However, these links did not clarify whether VEGF instigates or simply accompanies angiogenesis. To resolve that issue, Ferrara wanted to assess whether quelling VEGF obliterates formation of new blood vessels in a live creature. He thus sought ways to foil the protein in experimental animals.
Inactivating the Vegf gene provided one such method. In 1996, Ferrara’s group and a team led by Peter Carmeliet (Flanders Interuniversity Institute for Biotechnology, Leuven, Belgium) independently reported that disrupting a single copy of Vegf in mice caused embryos to die early in development. This result was surprising, as the embryos retained one operational copy of the gene. Furthermore, the finding indicated that other members of the VEGF family, which had surfaced by then, don’t compensate for even partial loss of VEGF. These observations indicated that the rodents are exquisitely sensitive to VEGF dosage and established the protein as a crucial player in embryogenesis.
To probe VEGF’s role in a mature animal, Ferrara harnessed the discovery of its receptor, which he made in 1992 with Lewis T. Williams (UCSF). This protein dwells on endothelial cells, where it binds VEGF and, in response, triggers cellular activities. Ferrara shortened the receptor so it would not associate with cells; instead it intercepted VEGF and impeded its function. In grown rats, the modified receptor almost completely wiped out angiogenesis in tissue that is normally loaded with blood vessels, Ferrara reported in 1998; furthermore, it prevented the tissue from performing its biological tasks. This study demonstrated VEGF’s importance in normal adult physiology.
Meanwhile, Ferrara had been pursuing other methods of inactivating VEGF, aiming to tackle disease. Toward that end, he developed a mouse monoclonal antibody that neutralizes human VEGF. In 1993, he reported that this agent foils the ability of several human tumor cell lines to multiply after they have been implanted into mice. These results provided the first direct evidence that tumor growth depends on angiogenesis and opened the door to clinical use of anti-VEGF compounds.
Envisioning better sight
In 1994, Ferrara turned his eye toward retinal disease. He and Lloyd Paul Aiello (Brigham and Women’s Hospital and Harvard Medical School) analyzed fluid from the eyes of patients with retinal disorders that stemmed from different underlying causes. VEGF appearance correlated with conditions that involve formation of new blood vessels, but not with other illnesses.
To test whether subduing VEGF might dispel eye diseases caused by pathological angiogenesis, Ferrara injected a derivative of the VEGF receptor into the eyes of mice with that type of retinal malady. The extent of new blood-vessel formation plunged in 46 out of 47 animals, he reported in 1995. The next year, with Anthony Adamis (Children’s Hospital, Boston), he conducted experiments on monkeys that suffered from a related eye condition. The same monoclonal antibody that Ferrara had used to obstruct tumor growth stymied blood-vessel formation in that setting. These experiments suggested that anti-VEGF agents might fight a human disease that also arises from inappropriate vascularization — wet AMD, a leading cause of severe, irreversible vision loss in the elderly.
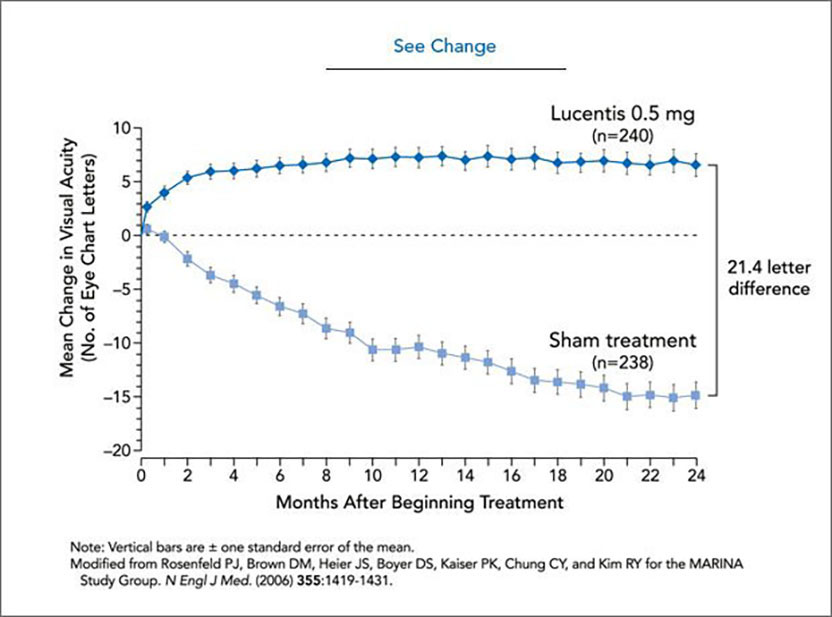
See change. Every month, patients’ eyes were injected with 0.5 mg Lucentis® (top curve) or prepared for treatment, but not injected (bottom curve). The drug improves average visual acuity, as can be seen by enhanced ability to read an eye chart. At the end of two years, the group that received Lucentis ® could see, on average, 21.4 more letters than the sham-treated group, which lost vision during that period. CREDIT: Cassio Lynm
Ferrara decided to groom the mouse monoclonal antibody rather than the soluble receptor for therapeutic use, in part because he knew that he could render it tolerable to the human immune system by engrafting the VEGF-binding portion onto a human antibody. Ferrara and his colleagues considered delivering the product — called bevacizumab — to the eye by injecting it into the bloodstream. They worried, however, about the systemic presence of an agent that interferes with blood-vessel formation in a population — elderly people — that has a high risk of cardiovascular disease. Therefore, the scientists chose to administer it directly into the eye. With that goal in mind, Ferrara and Henry Lowman (Genentech) developed a smaller derivative of the anti-VEGF antibody, ranibizumab, which they anticipated would penetrate the retina better than its parent, be more potent, and carry a lower risk of certain inflammatory responses.
In 2000, Ferrara and colleagues, including Philip J. Rosenfeld (University of Miami Miller School of Medicine) and David M. Brown (Methodist Hospital, Houston), began wet AMD clinical trials with this anti-VEGF antibody. Encouraging data led to multi-center trials on more than 1000 patients. The drug not only stops vision loss, but improves sight in many patients after a year of monthly injections (see graph); its effects persisted through the second year of the study. Other available therapies are modestly effective at halting disease and do not restore vision. On June 30, 2006, the US FDA approved ranibizumab (Lucentis®) for the treatment of wet AMD. Two years earlier, the parent compound (Avastin®) had been approved for metastatic colon cancer, and many individuals are receiving this drug ‘off label’ for wet AMD.
Ferrara’s work is revolutionizing quality of life for many of the estimated 1.2 million individuals in the United States who have wet AMD. Upwards of a million AMD patients worldwide have already received anti-VEGF antibody therapy. Many more will likely benefit in the coming decades, as disease frequency increases rapidly due to the world’s aging population.
by Evelyn Strauss
Key publications of Napoleone Ferrara
Ferrara, N. and Henzel, W.J. (1989). Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 161, 851-858.
Leung, D.W., Cachianes, G., Kuang, W.-J., Goeddel, D.V., and Ferrara, N. (1989). Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 246, 1306-1309.
Kim, K.J., Li, B., Winer, J., Armanini, M., Gillett, N., Phillips, H.S., and Ferrara, N. (1993). Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 362, 841-844.
Presta, L.G., Chen, H., O’Connor, S.J., Chisholm, V., Meng, Y.G., Krummen, L., Winkler, M., and Ferrara, N. (1997). Humanization of an anti-VEGF monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 57, 4593-4599.
Ferrara, N., Damico, L., Shams, N., Lowman, H., and Kim, R. (2006). Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 26, 859-870.
Ferrara, N. (2009). History of discovery: Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 29, 789-791.

 “A revolution is an idea which has found its bayonets.” These words, written by a military and political leader of Genoese ancestry, Napoleon Bonaparte, are exemplified in the work of today’s Lasker~DeBakey Award recipient, a basic and clinical science leader and another notable Napoleone of Italian ancestry, Napoleone Ferrara. For decades, scientists had the idea that there was a diffusible factor that promotes blood vessel growth and, in turn, that inhibiting this growth might be useful in the treatment of certain diseases. But the identity of this factor proved maddeningly elusive, and the concept remained untested. Napoleon Ferrera gave this important idea its bayonets by identifying and characterizing this factor and developing specific antibodies that prevent its function. Ferrara indeed started a therapeutic revolution by showing that inhibition of this molecule’s action is highly effective in preventing blindness in people with wet age-related macular degeneration (AMD).
“A revolution is an idea which has found its bayonets.” These words, written by a military and political leader of Genoese ancestry, Napoleon Bonaparte, are exemplified in the work of today’s Lasker~DeBakey Award recipient, a basic and clinical science leader and another notable Napoleone of Italian ancestry, Napoleone Ferrara. For decades, scientists had the idea that there was a diffusible factor that promotes blood vessel growth and, in turn, that inhibiting this growth might be useful in the treatment of certain diseases. But the identity of this factor proved maddeningly elusive, and the concept remained untested. Napoleon Ferrera gave this important idea its bayonets by identifying and characterizing this factor and developing specific antibodies that prevent its function. Ferrara indeed started a therapeutic revolution by showing that inhibition of this molecule’s action is highly effective in preventing blindness in people with wet age-related macular degeneration (AMD).

