The 15-month-old baby lying on an operating table at Johns Hopkins Hospital in Baltimore on November 29, 1944, weighed a mere 9 pounds, less than half the average for her age. Even more striking were the purple tinge of her skin and, as one of the surgeons in the operating room later wrote, the "deep, dark blue" coloration of her lips.
Taussig examines a baby in 1981.
Images courtesy of The Alan Mason Chesney Medical Archives, Johns Hopkins University
The child, Eileen Saxon, was one of the so-called blue babies who suffer from heart defects or other abnormalities that prevent the circulatory system from delivering enough oxygen. At the time, these children rarely survived long. However, Helen Taussig, a Johns Hopkins pediatric cardiologist, had envisioned a strategy to help babies like Saxon by replumbing their circulation. Albert Blalock, the hospital's chief of surgery, was about to attempt this correction for the first time.
As Taussig and a crowd of onlookers watched, Blalock made an incision between two ribs on the left side of Saxon's chest, beginning a four-and-a-half-hour operation during which he cut the artery that routes oxygenated blood to the left arm and plugged it into one of the arteries that transports deoxygenated blood to the lungs. Once blood began flowing through the re-directed vessel, Taussig announced, "Eileen's lips are a glorious pink color!" The healthier hue confirmed that Saxon's tissues were receiving more oxygen.

Blalock became chief of surgery at Johns Hopkins Hospital in 1941.
Images courtesy of The Alan Mason Chesney Medical Archives, Johns Hopkins University
The procedure that Saxon underwent that day, now known as the Blalock-Taussig shunt, provided only a temporary respite — she died after a second operation a few months later. But it has saved the lives of thousands of other children and earned Taussig and Blalock a share of the 1954 Lasker Award. The operation was also a landmark in surgery, showing that it was possible to reconstruct the heart and blood vessels to treat defects, says George Hines, a vascular surgeon at New York University/Winthrop Hospital who has written about the procedure's history. "It was momentous."
Mending Broken Hearts
Robert Gross, the other recipient of the 1954 Lasker Award, set the stage for the Blalock-Taussig shunt. A surgical resident at Boston Children's Hospital, Gross took aim at the ductus arteriosus, a vascular bypass that before birth diverts blood away from the lungs. The structure normally seals up shortly after a baby is born, but in some people it remains open. These patients are not blue babies, but they can suffer from shortness of breath and other debilitating symptoms, and they often develop heart failure.
In 1938, when Gross proposed sealing an unclosed ductus arteriosus in a young patient, heart surgeries were rare and risky. The hospital's chief of surgery forbade the operation. Gross performed it anyway, tying off the ductus arteriosus of a 7-year-old girl while the chief of surgery was on vacation. Although other surgeons had attempted this feat, Gross was the first to report a success. His patient survived until 2020. Gross' achievement was a milestone because he demonstrated that surgeons could intervene to repair a congenital heart flaw.
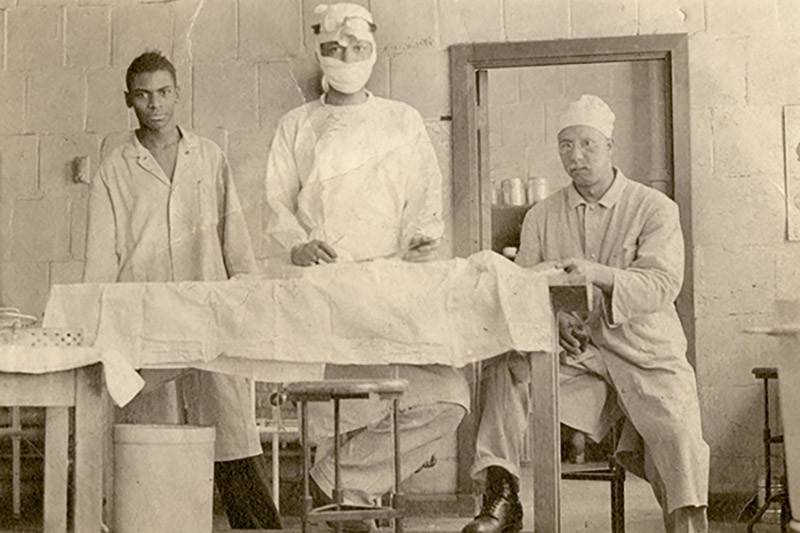
As Blalock's lab assistant at Vanderbilt University, Thomas (center) honed his surgical skills by operating on dogs.
Image courtesy of The Alan Mason Chesney Medical Archives, Johns Hopkins University
Developing the Blalock-Taussig shunt was a bigger step than closing the ductus arteriosus, says Hines. "This was a reconstructive operation nobody had thought about before." Taussig, who came up with the idea, had already distinguished herself. Undeterred by dyslexia and hearing loss, she had become head of Johns Hopkins's pediatric cardiac clinic, one of only a few such facilities in the United States. Taussig had examined many blue babies in her practice and had noticed that infants with an open ductus arteriosus seemed to fare better. She reasoned that engineering a similar diversion in the vessels near the heart could steer more blood to the lungs and increase the amount of oxygen the circulatory system distributes. The first person she asked to turn her insight into reality was Gross, but he declined, saying, "My job is to close ducts, not create them." Then she approached Blalock, who agreed to devise a surgical approach.

Thomas designed this clamp to close off the subclavian artery during the first "blue baby" operation in 1944.
All images courtesy of The Alan Mason Chesney Medical Archives, Johns Hopkins University
The procedure would not have been possible without Vivien Thomas, an African American assistant in Blalock's lab. Thomas mastered the surgical technique by operating on dogs, then worked with Blalock to adapt it for human patients. Because pediatric heart operations were novel, Thomas had to craft new instruments and pare the surgical needles so they would be small enough for stitching tiny vessels.
His experience proved vital during the operation on Saxon, during which he offered frequent advice to Blalock. "Vivien Thomas had done it over and over again in the lab," says Hines. Thomas's role was so important that many cardiologists now refer to the procedure as the Blalock-Thomas-Taussig shunt.

Unable to attend medical school in his youth, Thomas, shown here around 1970, eventually earned an honorary doctorate from Johns Hopkins University in 1976 and was appointed instructor of surgery.
All images courtesy of The Alan Mason Chesney Medical Archives, Johns Hopkins University
The collaboration among the three "was ahead of the times for the way these diverse people interacted and worked together," says Luca Vricella, chief of pediatric cardiac surgery at University of Chicago Medicine, who has performed around 200 Blalock-Taussig shunts, many of them when he worked at Johns Hopkins. The operation opened the way for a host of later corrective procedures, he says. "This is where pediatric cardiac surgery starts."
The shunt was a temporary fix, however, and many children needed further surgeries to have a normal lifespan. Today, infants with the same circulatory fault as Saxon typically undergo a different procedure that permanently repairs the problem. But surgeons still perform the Blalock-Taussig shunt on children whose hearts are missing a ventricle, Vricella says. With this help, "they can live well into their 20s and 30s."
Easier Breathing Through Chemistry
The winner of the 1994 Lasker Award, John Allen Clements, also tackled a problem that prevented some babies from getting enough oxygen. Even for full-term infants, the first breath is tricky. Not only do they have to draw air into lungs that are full of fluid, but the air sacs in their lungs can collapse when they exhale. Breathing is even more difficult for premature infants, who can develop a potentially fatal condition called respiratory distress syndrome (RDS). Clements's research helped to identify a molecular mixture in the lungs known as surfactant that stops the air sacs from collapsing, a discovery that spawned a new family of drugs for treating RDS. "They have saved the lives of millions of premature babies," says neonatologist Henry Halliday, an emeritus professor at Queens University Belfast in the United Kingdom.
The drugs indirectly emerged from Cold War work on nerve gas. In the early 1950s, Clements was a US Army doctor probing the effects of these chemical weapons on the lungs. To better understand how the lungs work, he and several other researchers independently began investigating the role of surface tension during breathing. Caused by clingy molecules at the boundary of a liquid, surface tension rounds raindrops and allows water striders to skate across ponds. Clements suspected that the lungs contained a surfactant that reduced surface tension during exhalation, preventing the air sacs from crumpling. The possibility was "remarkable," he said in a 2019 interview. But he had to find the evidence. By tweaking a standard lab apparatus, Clements was able to measure the surface tension in fluid he extracted from animal lung tissue, and in 1957 he confirmed the presence of a surfactant. "That was a key to fully understanding the cause of RDS," says Halliday. Just a few months later, two other researchers took the next step, analyzing fluid from the lungs of premature babies who had died from RDS. The newborns' lungs lacked surfactant.

Clements working in his lab at the University of California, San Francisco, in 2004
Image courtesy of Elisabeth Fall
Clements, who spent the rest of his career at the University of California, San Francisco, also helped turn surfactants into drugs to treat RDS — a process that took more than 30 years. He and his colleagues focused on developing artificial surfactants. The drug they eventually came up with, Exosurf, cut the death rate of premature infants by 50% in clinical trials, and in 1990 it became the first surfactant to receive approval from the US Food and Drug Administration.
More potent surfactants have since supplanted Exosurf, but the drugs remain essential for RDS treatment, says neonatologist Richard Martin of Case Western Reserve University Medical School. "Every large- or medium- sized neonatal unit would administer surfactants at least once a day."
Peace in the Womb
The winners of 1980 Lasker Award figured out how to spare babies' blood cells from destruction. John Gorman, one of the recipients, says that the discovery was not scientifically profound. "It was an idea that was out there waiting." But when he and four other scientists seized that idea in the early 1960s, they halted a health scourge that was killing 10,000 babies every year in the United States alone.

Freda (left) and Gorman
Image courtesy of Elizabeth Wilcox, Archives & Special Collections, Health Sciences Library, Columbia University
The problem, known as Rh disease, stems from an immunological clash between mother and fetus. About 94% of people have Rh-positive blood, meaning their red blood cells sport a particular protein called the Rh factor. People who are Rh negative lack the protein. If blood cells from an Rh-positive person enter the bloodstream of a person who is Rh negative, they trigger a reaction. The Rh-negative person's immune system pumps out antibodies that attack red blood cells carrying the Rh factor.
This incompatibility can become dangerous during pregnancy because some of a baby's blood cells infiltrate the mother's blood, often during birth. The first Rh-positive child of an Rh-negative mother usually will not suffer any ill effects, but the child's blood cells will sensitize her, prodding her to make antibodies that target the Rh factor. If she gets pregnant with another Rh-positive child, those antibodies will swarm across the placenta and begin destroying the baby's red blood cells, potentially causing anemia, heart failure, brain damage, and death.
Obstetrician Vincent Freda of Columbia-Presbyterian Medical Center wanted to find a way to prevent the reaction, and he asked Gorman, who was then head of the medical center's blood bank, to help.
Drawing on an observation from a 50-year-old paper, Gorman and Freda hypothesized that giving anti-Rh antibodies to an Rh-negative mother shortly after her child's birth would destroy any Rh-positive cells that had slipped into her blood, thus preventing her from becoming sensitized and protecting her future Rh-positive babies. "I realized this could be the solution to Rh disease," Gorman says.
Skeptics disagreed, however, and scoffed at the notion that "the medicine that was going to prevent the disease was the exact same thing that was killing the babies," says Gorman.

William Pollack
But he and Freda decided to test the hypothesis and asked a colleague, immunologist William Pollack, then at the Ortho Research Foundation in New Jersey, to create a preparation with high levels of anti-Rh antibodies, later renamed RhoGAM.

Cyril Clarke
Across the Atlantic Ocean, two other scientists had homed in on the same solution from a different starting point. Cyril Clarke was a physician and butterfly aficionado at the University of Liverpool in the United Kingdom who became interested in Rh factor because it was inherited through a similar genetic mechanism as the wing patterns of his favorite insects. Clarke and his student, Ronald Finn, followed up on a curious observation — babies were less likely to suffer from Rh disease if they had a different ABO blood group than their mother. The most likely explanation was that the ABO incompatibility spurred the mother's immune defenses to quickly dispatch any of the baby's cells that crossed into her circulation before they could elicit production of anti-Rh antibodies. Clarke and Finn suspected that injecting anti-Rh antibodies into the mother would do the same.

Ronald Finn
For safety reasons, the US and UK groups first evaluated anti-Rh factor antibodies in men, who also produce Rh-targeted antibodies when they are exposed to the protein. Both groups then established that the approach worked in Rh-negative pregnant women. The US researchers found that none of the 335 women who received the treatment carried anti-Rh antibodies 6 months after their first pregnancy. By contrast, 11% of women in the control group had these antibodies.
All five researchers shared the Lasker Award for coming up with this preventive measure. Rh disease continues to kill babies in less developed countries, where many women don't have access to the treatment or the blood type testing necessary to identify who is at risk. But in developed countries, the treatment made an immediate impact, Gorman says. "The results were incredible. In the developed countries, the disease just disappeared."
These are just a few of the Lasker-winning researchers and advocates who have helped improve the health of mothers and their children. Other issues Lasker Laureates have tackled include global child poverty, care of premature babies, family planning and infertility, and vaccines and treatments for childhood illnesses. Many of their contributions continue to save the lives of mothers and babies and improve health today.
By Mitchell Leslie



