John Gurdon clearly didn’t have what it takes to become a scientist, according to his biology master at Eton, the famous public school in the United Kingdom. Of the 250 students who took biology in the same term in 1949, Gurdon had gotten the lowest score. Pursuing a scientific career “would be a sheer waste of time, both on his part, and of those who would have to teach him,” the teacher wrote.

Gurdon holding the Lasker Award at the Ceremony in 2009
Gurdon soon proved that judgment wrong. As a graduate student at the University of Oxford, Gurdon performed a landmark embryology experiment to show that a mature cell could reset to an earlier developmental stage. “It was a huge breakthrough,” says developmental biologist Jose Cibelli of Michigan State University. Gurdon’s results launched animal cloning, valuable today for research and agriculture. His work also raised the possibility of reversing development in cells to produce treatments, an approach scientists are testing in various diseases. Gurdon’s research was so significant because “conceptually, it changed the dogma that development was a one-way street, and it made practical use possible,” says developmental biologist Yi Zhang of Harvard Medical School.
Unlike Gurdon’s biology teacher, others have recognized his remarkable talents. He shared the 2009 Albert Lasker Basic Medical Research Award and the 2012 Nobel Prize in physiology or medicine. A research institute at Cambridge University is named in his honor, and he was knighted in 1995.
A Rocky Beginning
Born in 1933, Gurdon grew up in a village about 70 km southwest of London. His parents were not scientists, but from an early age he was fascinated by insects and plants. His schools didn’t offer much science training—his first science class was the biology course at Eton, which he took at age 15. Gurdon floundered in the class, he said, because students had to copy down the teacher’s rambling lectures and memorize many facts. According to Gurdon, he took poor notes and doesn’t have a good memory. After his poor performance, the school didn’t permit him to take any more science classes, shunting him into “a course intended for those judged to be unsuited for studying any subject in depth,” he wrote.
Although Gurdon was still keen on science, after graduating from Eton he applied to study classics at Oxford. His mediocre entrance exam results nearly sank his chances of admission. He was eventually accepted partly because Oxford needed paying students, but the university stipulated that he not study the subject in which he was examined. He chose zoology instead. In retrospect, Gurdon said, being banished to nontechnical courses at Eton was beneficial because “I did not have to do the dreary kind of school science that people did have to do at the time.” Still, before starting at Oxford, he had to spend a year in an intensive remedial program.
Gurdon did well enough as an undergraduate that graduate school was an option. With his long-term interest in insects, he applied to the PhD program in Oxford’s entomology department. The department rejected him, which turned out to be one of the lucky breaks in his life, he said. At the time, entomology wasn’t pursuing profound scientific questions, Gurdon said. Instead, he joined a lab at Oxford that was probing developmental biology, a field where he could perform leading-edge research.
Turning Development Around
Shortly after starting his graduate work in 1956, he got the chance to tackle a problem that had stumped scientists for almost a century. A fertilized egg can produce any type of cell in the body. As embryonic development progresses, however, cells specialize, or differentiate, to perform particular tasks, and they lose that versatility. A fertilized egg spawns muscle cells and nerve cells, for example, but a muscle cell doesn’t transform into a nerve cell. Researchers didn’t understand how cells committed to a particular developmental path. The leading explanation held that cells differentiated by losing unneeded genes: muscle cells might jettison the genes required by skin or nerve cells, for example.
In the 1950s, two US researchers, Robert Briggs and Thomas King, tested that possibility by swapping out the nuclei of frog eggs. The scientists found that if they removed the nucleus of an egg and replaced it with a nucleus from a young frog embryo, the egg would develop into a healthy tadpole. However, if they replaced the egg’s nucleus with one from a slightly older frog embryo, development usually stalled. That difference suggested that cells changed irreversibly as they specialized, supporting the gene loss hypothesis.

African clawed frog, Xenopus laevis
Courtesy of ER Degginger / Science Source
At the recommendation of his graduate supervisor, Gurdon retried the experiment with a different frog species. Repeating a study by two respected scientists might seem like a waste of time, Gurdon said, but it made sense. Briggs and King could have been wrong, which would be useful to know. But if Gurdon confirmed their findings, the results might help researchers zero in on how cells differentiate. “Either way it turned out, it was going to be interesting,” he said.
Gurdon in the Lab in 2009
Throughout his career, Gurdon’s talent for lab work was one of the keys to his success, other scientists say. “He is a very good experimentalist,” says Marianne Bronner, a developmental biologist at the California Institute of Technology (Caltech). His experiments were always well-thought-out and designed to test specific questions, she says. In the lab Gurdon was meticulous and hands-on, recalls developmental biologist Edward De Robertis of the University of California, Los Angeles, who was his postdoctoral student from 1975 to 1980. Gurdon wrote extremely detailed protocols describing how experiments should be conducted. And even when he had assistants, he preferred to perform the procedures himself, De Robertis says.
When trying to redo Briggs and King’s work in the 1950s, Gurdon needed all his laboratory gifts. The species he was studying, the African clawed frog Xenopus laevis, was in some ways easier to work with than the North American species Briggs and King had used. For example, Xenopus can lay eggs all year round, whereas the North American frogs lay eggs only in the spring, limiting when researchers can run experiments. But Xenopus also posed some difficulties. The amphibian’s eggs are encased in an almost impenetrable tough coating, which prevented Gurdon from removing the nucleus from an egg and inserting a new one. In what Gurdon described as a stroke of luck, his supervisor had just bought a new ultraviolet microscope for the lab. Gurdon noticed that exposing the eggs to UV light could solve both problems, breaking down the tough coating and destroying the egg’s chromosomes so that he could replace them.
Now Gurdon could test whether Briggs and King were right. He started out by reprising their experiment, replacing the egg’s nucleus with one from an embryo cell that had begun to differentiate. He performed the procedure on multiple eggs—which sometimes developed into tadpoles, contradicting Briggs and King’s results.
Then Gurdon went a step further. He transferred nuclei from the intestinal cells of tadpoles into eggs. Unlike the embryonic cells he and Briggs and King had used before, the intestinal cells were specialized. If the gene loss hypothesis was correct, those cells would have shed the genes necessary to make other types of cells, and the eggs that received them would not develop normally. But Gurdon discovered that the eggs sometimes grew into tadpoles that became seemingly healthy frogs.
“It was the perfect elegant experiment,”
Cibelli says.
Each frog was a clone—an identical genetic copy—of the tadpole that had furnished the intestinal cell. Before Gurdon could say for sure that the frogs were normal, he had to find out whether they were fertile. However, when Gurdon finished his PhD, the frogs weren’t yet sexually mature. He left the experiment hanging and moved to Caltech for a one-year postdoc to investigate the genetics of bacteria-attacking viruses. When Gurdon returned to Oxford in the early 1960s, the university hired him as a lecturer, and he resumed working on the frogs. They could reproduce, confirming that the nucleus from a differentiated cell could spark normal development when placed into an egg. Gurdon reported those results in 1966.
His findings “showed that genetic information is retained [during development], which was really important,” Bronner says. The gene loss hypothesis was wrong. Researchers have since discovered that cells specialize by shutting down certain genes instead of throwing them out.
Cloning and Stem Cells
The impact of Gurdon’s discoveries went far beyond sinking the gene loss hypothesis. He was the first researcher to clone animals from differentiated cells. Scientists took more than 30 years to clone a mammal—Dolly the sheep, born in 1996, was the first—but now have performed the trick in more than 20 species. Researchers have used the technique to create more than 1,000 animal models for studying diseases, Cibelli says. In agriculture, cloning of cows and pigs with advantageous traits is common.
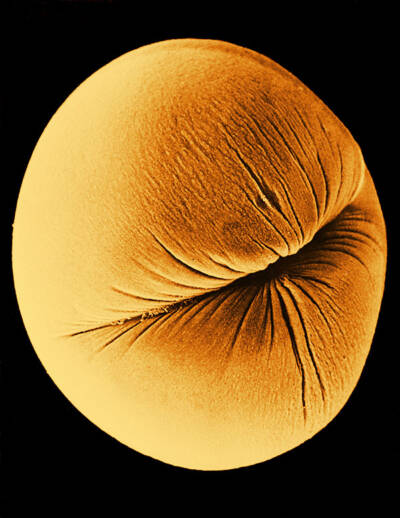
A scanning electron microscope image of the first cleavage of a clawed frog egg
Courtesy of David M. Phillips / Science Source
Gurdon’s work also was a watershed because it revealed that the developmental clock could run backward, with cells returning to an unspecialized state. The egg reprograms the nucleus, as scientists put it, allowing development to start from the beginning. That revelation, Zhang says, “means that we can reverse the developmental process” to produce undifferentiated cells that might be useful for treating diseases. For 40 years, the only way researchers could trigger reprogramming was by inserting cell nuclei into eggs. Then in the mid-2000s, stem cell biologist Shinya Yamanaka of Kyoto University found that adding four proteins to adult cells could spur them to revert to induced pluripotent stem cells (iPSCs), which are in an undifferentiated, embryonic-like state. Yamanaka’s results inspired a new appreciation for Gurdon’s findings, Cibelli says. Gurdon and Yamanaka shared the Lasker and Nobel Prizes. Researchers can now stimulate adult cells to transform into iPSCs and then coax them to differentiate, producing replacement cells to repair damaged or diseased tissues. Clinical trials are testing iPSCs in illnesses such as heart disease, Parkinson’s disease, macular degeneration, and diabetes.

Shinya Yamanaka and Gurdon at the 2009 Lasker Awards Ceremony
Researchers who know Gurdon say he stands out not only for his scientific abilities but also for his politeness, humility, and consideration for others. “He is a very proper man with very good manners,” De Robertis says. Gurdon also goes out of his way to help students, says De Robertis, who was a graduate student at the Leloir Institute in Buenos Aires, Argentina, when he met his future mentor in 1972. After a seminar, De Robertis gave Gurdon a ride to his hotel. To repay that kindness, Gurdon arranged a postdoc position for De Robertis in Britain. “It was an amazing gesture that nobody else would do,” De Robertis says. Cibelli adds that Gurdon has another important quality: grit. “He’s pretty resilient and a little bit stubborn. If he runs into a problem, he keeps at it.”
Gurdon kept at science for seven decades, working in his lab until the age of 90. His life would have been very different had he heeded the harsh report from his biology teacher at Eton. The lesson of his career, he said, is that “if you’re really interested in something, keep going. Don’t just give up because the teacher doesn’t think you are much good.”
By Mitchell Leslie

