Zhijian “James” Chen
UT Southwestern Medical Center
For the discovery of the cGAS enzyme that senses foreign and self DNA, solving the mystery of how DNA stimulates immune and inflammatory responses
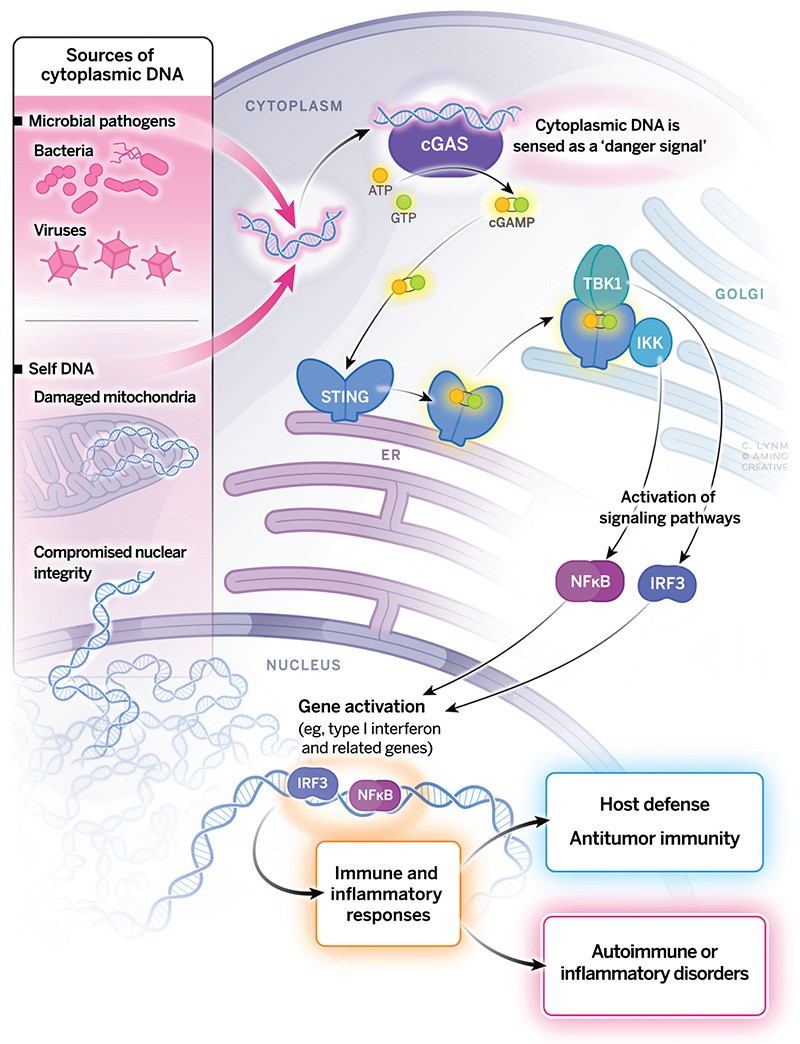
The 2024 Albert Lasker Basic Medical Research Award honors a scientist for the discovery of the cGAS enzyme that senses foreign and self DNA. With original thinking and tour-de-force experimentation, Zhijian “James” Chen (UT Southwestern Medical Center) solved the mystery of how DNA stimulates immune and inflammatory responses. cGAS underpins a major mechanism by which mammals combat microbial invaders and it fosters antitumor immunity. In certain physiological settings, inappropriate cGAS activity contributes to autoimmune and inflammatory disorders. The enzyme provides a pharmaceutical target for diverse human maladies, and the signaling molecule that it generates holds promise for fighting infectious diseases and cancer.
The boogie-woogie approach to creativity in art and science
The Dutch painter Piet Mondrian, famous for his geometric grid paintings, was an ardent jazz fan. In the late stage of his career, he became intrigued with boogie-woogie music, which inspired him to paint his masterpiece, Broadway Boogie Woogie.
Award Presentation: Michael Brown
Ordinary science advances slowly. We scientists move forward like a couple on a dance floor doing a slow box step. One step forward. One step to the side. One step back. Every so often a graceful couple captures the dance floor. Like Ginger Rogers and Fred Astaire, they sweep across the room. In a single step they twirl across a threshold, leading us ordinary scientists into a new ballroom where we can resume our slow box steps.
James Chen and his students are like Rogers and Astaire. In 2013 they published two elegant papers in a single issue of Science. The papers taught us how our bodies detect invasion by DNA viruses, and how this activates antiviral defenses. Biologists had crossed a threshold. Today we honor James Chen for his discovery and for the graceful elegance with which he made it.
Our bodies have two systems to defend against viruses. One is the immune system that generates antibodies. This system is powerful but slow. It takes time for antibodies to appear. So, the body needs a rapid defense to hold the line until the immune system can react. This rapid defense is called innate immunity. When a virus invades a cell, the cell secretes a protein called interferon. Like Paul Revere, interferon speeds through our bodies shouting, “the viruses are coming”. Other cells respond by mobilizing antiviral armies called cytokines. The cytokine army keeps the virus from advancing until George Washington can arrive with his antibody artillery.
A fundamental question is “how does a cell know it is infected, and how does this knowledge trigger interferon production?” Viruses come in two categories. Some use RNA as their hereditary material. Others use DNA. Our cells use different mechanisms to detect RNA and DNA viruses. In earlier work James Chen helped unravel the sensors for RNA viruses, but that is not the discovery that we honor today. Today, we honor Chen for showing us how our cells recognize DNA viruses—the viruses that cause smallpox, chicken pox, hepatitis B, cervical cancer, and many other diseases.
Most Lasker Awards recognize the thing that was discovered. In Chen’s case we recognize not only WHAT he discovered, but HOW he discovered it. His methods are original and breathtaking so I must describe them in a little detail.
In the first of their two Science papers, Chen and his students, Lijun Sun and Jiaxi Wu, invented a clever system to use biochemistry to discover the sensor for viral DNA. They used animal cells in tissue culture. To activate the sensing mechanism, they introduced foreign DNA into the cytoplasm of one set of cells. Then they made extracts from the DNA-treated cells and added the extracts to other cells that were permeabilized to allow the signaling molecule to enter. They found that the extracts from the DNA-treated cells produced a substance that activated the interferon gene in recipient cells that had not seen DNA. They assumed that the signaling substance was a protein, and their next task was to purify it. Soon they discovered that the signaling substance was not a giant protein but a tiny chemical—a combination of two molecules called AMP and GMP linked together in a circle to form Cyclic AMP-Cyclic GMP. They named it cGAMP. Molecules like cGAMP had never been seen before in animal cells.
The discovery of cGAMP alone would be worthy of a Lasker Award. But Chen and his students went much farther. Their first paper told us that cGAMP warns that viruses are invading. But how does viral DNA trigger cells to produce cGAMP? They solved this problem in their second paper, whose biochemical virtuosity is even more breathtaking than the first.
Chen and his students set out to purify the enzyme that detects DNA and makes cGAMP. Their only source was tissue culture cells. Instead of adding DNA to cells, this time they first made extracts of the cells and then added DNA to directly activate the enzyme that produces cGAMP. To detect cGAMP they added the activated solution to permeabilized cells that had not seen DNA, and again they measured interferon activation. To isolate the enzyme from the DNA-treated cell extracts, they separated the proteins into fractions and incubated each fraction with DNA to see if that fraction contained the enzyme. Here they faced a problem. Tissue culture cells contain tiny amounts of protein. To identify the enzyme, they had to separate it from the many thousands of proteins in this tiny amount of starting material. They used standard methods of protein purification, but their most purified solution still contained too many proteins. They couldn’t tell which protein was the enzyme. They tried two other methods to purify the enzyme and again each purified solution contained too many proteins. Then they hit upon their brilliant idea. Each of their three purified solutions contained a different mixture of proteins, but they all produced cGAMP so they all must contain the cGAMP enzyme. The cGAMP enzyme might be the only protein that was present in all three purified preparations. So, they used mass spectrometry to identify the proteins. Only three proteins were present in all three solutions. When they analyzed the sequence of these three proteins, the correct one was obvious. One protein resembled known enzymes that work on nucleotides. This must be the culprit. They named their enzyme cGAS which stands for cGAMP synthetase.
Using recombinant DNA techniques, they produced pure cGAS. They showed that DNA binds to cGAS and activates it to produce cGAMP. But there was a problem. cGAS is activated by animal DNA as well as viral DNA. How does it distinguish the two? The answer lies in real estate. Location. Location. Location. cGAS is in the cytoplasm where viral DNA is located. Animal cell DNA is restricted to nuclei or mitochondria. cGAS is activated only by cytoplasmic viral DNA.
Once Chen had crossed the DNA sensing threshold it was time for the box steppers to step in. Chen and the box steppers showed that cGAMP binds to a protein called STING that transmits the signal to activate the interferon gene. The implications for medicine are profound. To increase the immune killing of cancer cells, pharmaceutical companies are testing drugs that activate cGAS. On the other hand, cGAS is sometimes activated abnormally by cellular DNA that leaks out of the nucleus. Activated cGAS produces cGAMP which triggers interferon production and causes inflammation and tissue damage. The result is autoimmune diseases like lupus and rheumatoid arthritis. Drugs that inhibit cGAS are effective in animal models of autoimmune diseases. Soon trials in humans will begin.
In summary, James Chen is the Fred Astaire of viral immunity. He is a worthy recipient of the Lasker Basic Science Award.
Acceptance remarks: James Chen
As a kid growing up in a remote mountain in southern China, I often looked up and gazed the stars. But never had I ever imagined that I would be gazing at my stars so closely, now at the Lasker Luncheon. I want to thank the Lasker Jury and the foundation for selecting me to receive this tremendous honor.
When I was young, I learned that the wind is created by mixing hot air and cold air. Based on this, I drew a wind machine, attempting to blow away the mountains surrounding me, because I thought the mountains kept us poor and isolated. After living in Dallas for nearly three decades, now I miss those mountains. Obviously, my wind machine never worked, but my parents found a better way, which was to send me to school. Now I am living proof that education is the greatest equalizer. And my message to the young people is this: if I can get so close to the stars, you can too.
My love for science, and for biochemistry in particular, was aroused by the late Cecile Pickart, my PhD advisor who taught me the art of protein purification. My interest in applying biochemical approach to solving biological problems was encouraged by Tom Maniatis, who taught me how to choose important problems to work on.
In 1997, I started my lab at UTSW, right next to the lab of Eric Olson, who recruited me to join the new department of Molecular Biology. This was the best move in my life, because UTSW is a scientific oasis built and cultivated by my scientific heroes and leaders who are here today. In Dallas, I was fortunate to recruit talented students and postdocs who believe in the power of biochemistry in making discoveries even in this post-genomic era. This effort culminated in the discovery of cGAS and cGAMP by a dream team led by Josh Sun and Jiaxi Wu. It has been gratifying to see the rapid progress of understanding the cGAS pathway in the past decade, thanks to spectacular efforts by many scientists around the globe.
Today I feel honored to receive the Lasker award for basic research together with the clinical awardees who discovered GLP1 and developed the medicine that has benefited millions of people. It is my dream that one day our discovery of cGAS will have a similar clinical impact.