By many accounts, there has never been a better time than now to do biomedical research.
Scientists can take advantage of the multiple technologies that came of age across multiple fields around the turn of the 21st century — from the advent of fast and inexpensive DNA sequencing to the leaping bounds in data science and computing power to the explosion of imaging tools. Such sweeping advances, combined with decades of painstaking molecular breakthroughs, have made it possible to determine the structure of proteins within cells for the first time, to develop possible cures for sickle cell and related diseases using CRISPR genome editing, and to build on a long record of molecular biology and vaccine research to produce COVID-19 vaccines in less than a year.
“At this point in time, you are really only limited by your creativity and your boldness,”
says Kit Pogliano, dean of biological sciences at University of California, San Diego. “You can answer just about any biological question, and the tools are developing so rapidly, especially if you love to collaborate across different disciplines,” she adds. Collaborations across different sectors — academia, the biotech and pharmaceutical industry, philanthropic organizations, and communities of patients — are also more compelling than ever before.

NIH Building One under construction, 1938
The first building constructed at the newly formed National Institutes of Health, Building One began to take shape in 1938. President Franklin Roosevelt dedicated the National Institutes of Health in 1940 at the top of the stairs leading to the entrance. Building One, later named the James H. Shannon building in honor of NIH’s director from 1955 to 1968, now houses the Office of the Director among other administrative offices.
Courtesy of National Institutes of Health
Biomedical research has arrived at this moment globally in no small part because of the ongoing federal funding and fierce advocacy in the United States. “We are seeing the fruits of 75 years of steady investment at the National Institutes of Health (NIH) that the Lasker Foundation has been promoting,” says Elias Zerhouni, who directed the NIH from 2002 to 2008 and currently serves on the Lasker Foundation Board of Directors.
In some ways, the dream of Mary Lasker, who founded the Lasker Foundation with her husband Albert in 1942 as a medical research advocacy group, has been realized. Lasker’s dedicated lobbying of Congress to prioritize medical research put the NIH budget on a mostly upward trajectory starting in the late 1950s. The agency became a model for countries around the world setting up their own national systems for funding science. Prior to World War II, biomedical researchers had to rely primarily on funding from state governments or private philanthropic groups, such as the Rockefeller Foundation.
But many warn that the US is now in danger of losing its edge — not just in biomedical science but in all science and technology (S&T). The nation’s investment in research and development has been hovering around 2.5% of its GDP (gross domestic product) for the last few decades, whereas the amount of GDP invested into S&T by other countries, particularly China and Korea, has been on the rise, as outlined in a 2020 report, The Perils of Complacency, by the American Academy of Arts & Sciences (AAAS) and Rice University. The report cautions that the United States is at a tipping point for becoming less competitive with countries such as China in its S&T research enterprise, and thus in its ability to create jobs and improve healthcare and overall quality of life.
According to Zerhouni, who was part of the committee that created the AAAS report, the most pressing need is to increase federal funding of physical sciences, especially federal agencies such as the National Science Foundation (NSF). Although the NSF does not fund biomedical research directly, it supports all other S&T disciplines, including other biological sciences. These fields have made enormous contributions to biomedical science and are critical to interdisciplinary or convergent research.
There is no question that the stakes are high. “We are in two global competitions right now — one is a competition against China and India and Europe,” says Sudip Parikh, chief executive officer (CEO) of the American Association for the Advancement of Science. “The second competition that we are in is against time, against the economic tidal wave that is coming from Alzheimer’s, cancer, and aging [diseases],” he adds.
Same Mission, Updated Strategies
The mission of the NIH, NSF, and other federal agencies has remained steadfast since the middle of the 20th century, when the US government committed to continuing its wartime support of scientific research. These agencies aim to promote scientific progress and support advances and discoveries that improve health and prosperity. That may sound ambitious, but the NIH can point to many examples of how it has fulfilled its mission over the decades — the most poignant of all may be its response to the COVID-19 pandemic.

Three NIH directors, Elias A. Zerhouni (left), Francis Collins (middle), Harold E. Varmus (right) led the last 28 years of the federal agency.
Courtesy of Chia-Chi Charlie Chang
The good news in recent years is that the NIH budget for funding extramural (not conducted on NIH campuses) biomedical research has been rebounding — growing 2% to 5% annually since 2014, following a 10-year stagnation — allowing the agency to diversify the types of research programs and teams it supports. However, these increases still do not allow the agency to award as many research grants as in the past — the success rate of NIH grants in 2020 was 21%, compared with 31% in 2003. ACT for NIH, a biomedical research advocacy organization, recommends that the NIH budget increases 10% in 2022, which would finally make up for inflationary loss since the early 2000s. In the longer term, ACT for NIH is trying to make the case to members of Congress to fund another NIH budget doubling (as Congress did from 1998 to 2003), arguing that the agency would then be able to support research at the scale needed to turn the many recent technological advances into medicines, such as immunotherapy for cancer and mRNA-based vaccines for infectious disease.
Despite its unwavering mission, the NIH has made some tweaks in how it operates over the years. A recent change was inspired by the rapid progress that NIH-funded sciences made during the pandemic, dissecting the SARS-CoV-2 virus and paving the way for diagnostics, treatments, and vaccines. “Never before have we seen research move to practice at this fast a clip,” says Marina L. Volkov, director of the NIH Office of Evaluation, Performance, and Reporting. “What can we learn from the pandemic; what were the active ingredients?” she says.
There will be a stepped-up focus on self-assessment of the NIH’s impact in the upcoming NIH Strategic Plan (2021–2025). There will also be greater emphasis than in the previous plan (2016–2020) on supporting the scientific workforce and physical infrastructure such as research facilities.

Undergraduate Angela Jimenez (foreground right) and PhD student Joscelyn Mejias (foreground left) conduct National Science Foundation-supported research at Georgia Tech to develop therapeutic cells. The lab is one of four NSF Engineering Research Centers working to develop new technologies that support health and energy research.
Courtesy of Rob Felt, Georgia Tech
Similar to the NIH, the NSF has been making adjustments in how it strives to achieve its mission. The agency, which accounts for about 2% of the federal investment in health and medicine research and development, compared with the NIH’s 82%, has been working to bolster funding for convergence research since the 1990s, through mechanisms such as Big Ideas and RAISE grants. “I think you’ll see more and more of that coming,” says Theresa Good, a program director in the NSF Biological Sciences Directorate.
Divvying Up the Pie: How to Make Research Support Fairer?
Many have sounded the alarm in recent years that the NIH needs to combat the growing disparity in research funding. Recent reports have found that 40% of NIH research dollars go to only about 10% of academic investigators, and they tend to be senior investigators at prestigious research institutions. One idea floated in 2017 and quickly thrown out, was to cap the number of R01s — the NIH’s most common type of grant — that any one investigator could have to free up money for other investigators.
However, some point out that ambitious research costs more than ever, and investigators need more grant money than ever. Many projects today aim to synthesize the myriad molecular discoveries of previous decades about individual components of a biological system, such as single genes or proteins. “We’ve gone from a reductionist phase to integrationist [approaches] where we integrate all the knowledge,” Zerhouni says. This kind of research can be expensive because it involves big groups, often coming together from different disciplines, such as computer scientists and engineers working with biologists.
The rising cost of doing biomedical research can make things even more stressful for academic scientists, who generally have to apply every few years for new or renewed grants. The philosophy of the Howard Hughes Medical Institute (HHMI), a US-based philanthropic medical research organization, which provides long-term support to over 250 US-based investigators, is to invest in “people, not just projects” and to do so based on the investigators’ track records. This type of support frees scientists from the pressure of regularly applying for grants, says David Clapham, vice president and chief scientific officer. As a result, they can pursue fundamental, less application-focused questions, which must nevertheless be answered before we can find disease treatments. Centers such as the Stowers Institute in Kansas City, MO, and the Scripps Research Institute in La Jolla, CA, and Jupiter, FL, can also accomplish this goal, Clapham says, because they have large endowments and let scientists loose to explore basic biological processes.
One of the newer types of NIH grant mechanisms, MIRA (short for Maximizing Investigators’ Research Awards), which was launched by the National Institute of General Medical Sciences (NIGMS) five years ago, seems to take a page out of the HHMI playbook: It provides longer-term funding, and thus more stability and research freedom. Each NIH institute has leeway to decide how to spend its congressionally determined piece of the NIH budget pie. Although other Institutes have created HHMI-like mechanisms, MIRA is special because it limits the amount of NIGMS funding that an investigator can receive, similar to the NIH-wide quota idea in 2017.
Following the Money: Funding from Industry and Philanthropy
For scientists pursuing biomedical research with possible clinical applications, there are increasing opportunities to get funding from industry and philanthropic groups and move discoveries beyond the walls of academic labs. The biggest gains in spending on health and medical research in the United States in recent years have come from industry, namely pharmaceutical and biotech companies, according to a recent report by Research!America, a science advocacy organization.
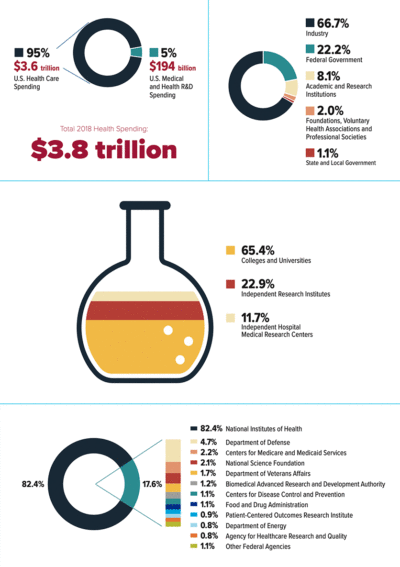
Following the money
Top left: R&D investment as a percentage of overall US health spending (2018)
Top right: US medical and health R&D expenditures by funding source (2018)
Middle: Academic and research institution investment in medical and health R&D, by funding sector (2018)
Bottom: Federal investment in medical and health R&D, by funding sector (2018)
Courtesy of Research!America
This sector actually accounted for 67% of spending in 2018, most of it supporting clinical and applied research, while 22% came from federal agencies, overwhelmingly from the NIH. Part of the reason for the rising investment from industry is that “larger [pharmaceutical] companies aren’t doing as much of the research themselves internally as they used to and so… they are looking more to academia to fuel the very earliest part of the research cycle,” says Paul Roben, associate vice chancellor of innovation and commercialization at University of California, San Diego. A company might fund an academic investigator’s entire research program in an area, such as immuno-oncology therapies, for a set period of time with clear milestones, which might be the discovery of a novel compound with certain characteristics, and then create a biotech or startup company to take the compound into early-stage clinical trials.
To help bolster the biotech stage of the academia-biotech-pharma life cycle, President Joe Biden has proposed creating a new NIH organization called ARPA-H (Advanced Research Projects Agency for Health).
“For small companies, the biggest and riskiest part is when they transition from a lab concept into product development, and that involves a lot of technology development and bioengineering that is really critical,”
as was the case for the development of COVID-19 vaccines, says Gary Nabel, president and CEO of the biotech company, ModeX Therapeutics, and former director of the NIH Vaccine Research Center. ARPA-H aims to fund the most ambitious of these types of projects, which private investors tend to shy away from. The agency’s success will depend on the budget that Congress allocates and how it decides to award grants. Nabel thinks that a model similar to that of the Department of Defense’s DARPA (Defense Advanced Research Projects Agency) could work well, in which federal and industry stakeholders agree on business plans that include a set of milestones.
The philanthropic sector contributes a much smaller piece of the pie of health and medicine investment in the United States, only about 2%, but as many point out, it is growing. And it can have outsized influence, particularly for large private foundations, such as the Bill & Melinda Gates Foundation, Chan Zuckerberg Initiative, and Simons Foundation. Philanthropy is once again providing critical support for science research, just as it did before the United States began committing federal funding in the mid-20th century, says France A. Cordova, president of the Science Philanthropy Alliance.
Overall, the interests of philanthropic individuals and groups run the gamut from basic to translational to clinical research, but one generality holds true:
“there’s a higher tolerance for risk”
says Melissa Stevens, executive director of the Milken Institute Center for Strategic Philanthropy. Stevens co-founded the Center in 2015 to help private foundations, as well as individuals, families, advocacy groups, and public charities, decide how to strategically invest in research on health, medicine, education, and the environment. Philanthropists may want to support basic research to tease out the biological underpinnings of a poorly understood disease to de-risk that field of study, she explains. Or they may want to support otherwise underfunded preclinical or early clinical research in academic labs and biotech companies, with the hope of generating data to entice pharmaceutical or venture capital investment, she explains. As Cara Altimus, Stevens’s colleague at the Milken Center, notes, philanthropy can be particularly successful in this so-called Valley of Death space, often by infusing a small biotech company with research support to advance a discovery along the development pipeline.
What Does the Future Hold for US Biomedical Research?
Despite concerns that the United States is not doing enough to maintain its S&T powerhouse status or to maintain its competitive edge with China and Korea, there may be some reasons for optimism. “I am actually pretty bullish that we are going to see much larger increases for NIH this year,” says Parikh. That is because budget sequestration is over, and Congress appreciates more than ever the need to support science to stay competitive with other countries and be positioned to find the next big thing — whether it is a new genome-editing technology or a platform for rapid vaccine development for the next pandemic.

Alondra Nelson, Harold F. Linder Professor in the School of Social Science at the Institute for Advanced Study and President of the Social Science Research Council (SSRC), has been appointed to the position of Deputy Director for Science and Society in the Office of Science and Technology Policy. Nelson will be the first person in this role, which brings social science expertise explicitly into the work of federal science and technology strategy and policy.
Courtesy of Ragesoss
In another positive sign that the US government is prioritizing science, President Biden elevated the director of the Office of Science and Technology Policy (OSTP), Eric Lander, to a Cabinet position, a first for the White House. One of the major goals of the OSTP is to improve the diversity of the scientific workforce. As Lander explained in an interview after he was sworn in, he and Alondra Nelson, who is creating a science and society division within the OSTP, will talk with various groups about solutions to “have everybody at the lab bench.”
It will be important, according to Parikh, to put more emphasis on how the next generation of scientists will be trained to communicate their work with the public.“We are going to have 100,000 scientists who are 35 and younger who are going to be participating in their communities and building bridges with policymakers,” Parikh says.
The importance of advocacy is one of the many legacies of Mary Lasker. Starting in the late 1940s, she and her partners recruited researchers and clinicians and prepared them to make their case at congressional budget hearings for research funding. “Doctors aren’t used to selling anything,” Lasker explained, but as she emphasized, they need to step into the role because the future of biomedical research is at stake.

by Carina Storrs
