When the secretary of the Nobel committee explained the award banquet to Michael Brown and Joseph Goldstein, he told them that each category has three minutes to make an acceptance speech. Which one of the 1985 winners would talk?
“Can’t we both?” they responded. Taken aback, the secretary told them that such a thing had never been done in the prize’s 75-year history. The pair persisted: “You’re about to destroy the very partnership that you’re honoring.”
Brown and Goldstein are sailing together.
Two weeks later, they heard back. “Each of you gets one-and-a-half minutes.” Slicing the time in half might have produced a choppy message, but Brown and Goldstein have a knack for pithy communication. In Stockholm, Goldstein conveyed an incisive truth about the power of their alliance in his first 30 seconds. “Some may think that we are too young for this award,” he said. “But let me point out that we work as a team. If our efforts were only additive, our combined age would be 45 plus 44, or 89 years. But our efforts are more than additive: they are synergistic. They have a multiplying effect. Our true collaborative age is 45 times 44, or 1980 years — surely old enough for a Nobel Prize.”
Thirty-five years after Goldstein explained these mathematics, Eric Olson, a cell biologist at the University of Texas Southwestern (UTSW) Medical Center in Dallas, independently describes their union in a similar way. “Either one of them would be the greatest scientist you ever met,” Olson says. “You put the two of them together, and the whole is greater than the sum of its parts.”
For almost five decades, Brown and Goldstein have applied their outsize clout to decipher how cells govern cholesterol metabolism. The unprecedented longevity of their professional marriage is outshined only by its creative productivity. Relentless curiosity, exceptional intelligence, and imaginative open-mindedness have fueled their experimental forays. Rigor and thoroughness have infused their findings with heft and fortitude. They have brewed a relationship that has spawned not only legendary discoveries, but a collaborative model that offers an inspirational paradigm.
“Those guys are a wonder to behold,” adds Olson.
“They’re the Lennon and McCartney of science without the rivalry.”
Breaking the curve
Goldstein grew up in Kingstree, South Carolina, whose population was 3182 in 1940, when he was born, and 3328 in 2010. Train tracks separated the Black and white sections of town, and young Joe attended segregated schools. Goldstein’s family was well established in the community. Both of his parents had gone to college, and his father held prominent public service roles. Furthermore, he ran Silverman’s, the town’s largest department store. Forty miles away, Goldstein’s mother’s clan also owned a shop — for women’s specialty clothing. “The department stores in small towns of the South were owned by Jewish families,” says Goldstein, although his was not particularly religious.

Goldstein’s balance served him on the see-saw and ever since.
After the elder Goldstein came home from work, he would quiz Joe on vocabulary. “That was always fun,” says Goldstein, who loved words and was “a compulsive reader.” Compton’s Encyclopedia sat on a shelf in the den, and “over three or four years, I probably turned every page,” he says. He looked forward to each Friday’s installment of Weekly Reader, a newspaper for elementary school children.
His mother, too, relished language and cultivated her son’s interest and aptitude. She “read all my high school essays and corrected the grammar,” says Goldstein. As a young boy, he invaded her library and borrowed many classics “that were not for children who were 7, 8, or 9 years of age,” he says. “They weren’t the Hardy Boys.” A couple of years later, he tackled The Caine Mutiny, Herman Wouk’s 500-page WWII historical novel. “That was one of my favorites,” he says. The courtroom drama captivated him, and he still remembers Captain Queeg’s steel worry balls.
When Goldstein was in fourth or fifth grade, his teacher phoned his father. She was hesitating to give Joe the B– he deserved, she explained, because the senior Goldstein served on the board of education. He told her she should issue a C if Joe deserved a C and then advised his son, “If you want to succeed in life, you better do your homework better,” says Goldstein. Within several years, the future Nobel Prize winner became an A student.
Goldstein was well-liked and respected. His peers elected him president of the class the last three years of high school. As a junior, he took the helm of the school newspaper, called The Boll Weevil, after a beetle that destroys entire cotton fields. Under Goldstein’s leadership, the publication lived up to its name, vanquishing much bigger schools in state competition.

First brush with celebrity, Goldstein interviewed financier Bernard Baruch (1958).
As editor, Goldstein had the opportunity to interview financier and presidential adviser Bernard Baruch, who owned a hunting lodge about 10 miles from Kingstree, where he shot quail and entertained celebrities such as the Roosevelts and Winston Churchill. “He was one of the few people who didn’t lose his money in the Depression, and everyone respected him because of that,” says Goldstein. “It was a big thrill to be able to talk to a national personality.”
While retelling this story 62 years later, Goldstein scans the article and notices Baruch’s hearing aid in the accompanying photograph. Its battery, whose wire is visible, “was the size of an iPhone,” says Goldstein, who often seems to be looking through the lens of research advances. “That shows you the progress in biomedical science.”
Although he grew up in the South, Goldstein surrounded himself with “people who were moderate or liberal,” political sensibilities that his parents transmitted. One time when Eleanor Roosevelt came to visit Baruch, Goldstein headed to the train station to watch the hoopla. Five or six Black musicians played the Star-Spangled Banner, he recalls. A small group of white people — “presumably the Ku Klux Klan, though they weren’t wearing hoods” — threw bananas, says Goldstein. “This is a terrible thing,” his parents told him. “People have prejudices that we don’t have.”

Goldstein enjoyed editing his high school newspaper, an experience that provided early training for writing scientific papers and chairing the Lasker Medical Research Awards Jury (1958).
His teachers were women, except for those who taught Latin and shop, and they were excellent. His chemistry instructor “explained common things you took for granted, like the moon and the sun and the stars. It was exciting and stimulating and challenging.”
During the summer, Goldstein visited an older cousin, Samuel Warshauer, at his beach house. Warshauer and his wife were accomplished bridge players, and they taught their guest the game. After Goldstein got home, he watched his mother and her friends, who had a bridge club that rotated among the members’ houses. As they played, he thought things like, “You should have bid 1 notrump.” The well-bred youngster kept such opinions to himself.
Goldstein majored in chemistry at Washington and Lee University in Lexington, Virginia. He was valedictorian of his class, yet academics did not consume him. He chaired the student library committee, for instance, and was editor in chief of the yearbook. Sometime in college, Goldstein decided to become a doctor. “I didn’t have a burning passion to treat patients at that point,” he says, although Warshauer, an internist, probably influenced him. “Early on, it was more that I didn’t want to be a lawyer.”
When Goldstein was pondering where to go to medical school, he mentioned UTSW to Warshauer. Joe had learned about this new institution from a fraternity brother. As it happened, Warshauer had recently attended an American College of Physicians meeting and had heard Donald Seldin and Morris Ziff, both of UTSW, give presentations. “He said they were the best talks at the meeting,” Goldstein says.
He was the first out-of-state student that the institution accepted, and he shone. Although he did not show off, his grades attracted attention. On exam days, one of his classmates always ribbed him: “Don’t screw us up today; don’t break the curve,” says Goldstein. His talents extended into the wards, where he presented patients to his instructors and peers “in a crisp way that stood out,” in part because he picked up on physical findings “that third-year students wouldn’t be expected to get.” In small group settings, the attending physician asked him questions after the other four or five students had their chance because “he knew I would probably have the answer.”
After Goldstein’s first year, he undertook a research project. In just a few months, he developed an improved method for assessing liver function and got his first experimental buzz. By the time he graduated, he had published four papers and received the Ho Din Award, the school’s “foremost honor bestowed on outstanding seniors” for compassion as well as knowledge.
Seldin not only gave impressive seminars; he also had a knack for spotting talent, and, as one of UTSW’s luminaries, he aimed to build the center into a preeminent medical enterprise. He picked bright students, sent them away, and then brought them back. Toward that end, he mapped a plan for Goldstein that would deliver the exceptional student to Dallas after rounding out his training at some of the country’s top institutions. He sent Goldstein off to do an internship and residency at Massachusetts General Hospital (MGH), a clinical fellowship at the National Institutes of Health (NIH), and then a postdoctoral fellowship in medical genetics, a specialty that barely existed at the time. Thus polished, the protégé would return to launch a medical genetics division at UTSW.
In Goldstein’s MGH class of 12 people, six were Harvard graduates. One of the other out-of-towners came from Pennsylvania.
Making waves in multiple milieux
When Michael Brown saw the list of his internship classmates, he wondered why the esteemed program had accepted someone from a Texas bible college. Brown “didn’t have high hopes for meeting this fellow,” he told an interviewer for the Nobel Foundation, but “It was obvious within a day or two that [Goldstein] knew more than most of the residents at the Mass General.”
Early on, Goldstein was part of a team that consulted on one of Brown’s patients. After Goldstein examined the man, he paged Brown. “He is going to crash tonight,” Goldstein said, according to Brown, who responded, “I don’t know. He looks pretty good.” Sure enough, the patient crashed. This experience clinched Brown’s respect for his colleague from the unknown Texas medical school.
Brown was born a year after Goldstein in Brooklyn, NY. At age 11, he moved to a Philadelphia suburb. “I had a very, very standard New York-Philadelphia kid’s existence,” Brown says. “I played a lot of stick ball and followed baseball.” He rooted for the New York Giants.

Amateur radio sparked Brown’s passion for the scientific method (1955).
In junior high school, a friend introduced him to amateur radio, and he began building transmitters and receivers so he could communicate with radio operators throughout the world. He learned how to work a telegraph key and mastered Morse code.
With his pals, Brown entered contests in which the participants aimed to contact the most people in Europe or Asia in 48 hours. “Four boys would gather in somebody’s house to test who had the most powerful transmitters and receivers. We’d do shifts.”
The experience, Brown says, groomed him for a career in science. “You’d build a transmitter and it would take a couple of weeks.” Integrated circuits didn’t yet exist, so “there were many parts — vacuum tubes and resistors and capacitors. I’d finish around 3 in the morning and plug it into the wall and promptly blow every fuse in the house. That’s where the science would begin. You’d have to figure out what you did wrong. Check every circuit. Find the wire I had soldered into the wrong place.” That kind of troubleshooting “is basically what I’ve done in the lab for my entire career. Was the theory wrong or did I make a stupid mistake in carrying out the experiment? How do I fix it?”
Brown’s parents did not have much money. Although they scrimped on expenses, his mother doused him with love. “She was a very intelligent woman who was my biggest fan,” he says. “She was cultured and literate, and she understood me much more than my father did.” When Brown entered high school, his mother penned a letter about him to the principal that oozes devotion and affection. “His sister Susan…thinks he is just about perfect,” she wrote, before offering this insight: “His best school work is done when he knows he has good competition, as he enjoys anything with an element of challenge.”
In high school, Brown became the sports editor for his school newspaper. “Sports reporting is the best training you can do for writing,” he says. “You have to think about novel ways to describe things” to avoid falling into clichés.
Brown’s dad was a textile salesman, and young Michael sometimes accompanied him on client visits. The dress manufacturers would “give my father hell,” Brown says. “‘The color of this fabric is not right and we’re going to have to return it.’ My father had to grovel.”
Brown wanted to be a physician for as long as he can remember, in part because his dad kept saying that the only people he knew who didn’t have a boss were doctors, “so he wanted me to be a doctor — a Park Avenue doctor,” Brown recalls. “If you were a Jewish kid in Brooklyn growing up the way I did, the highest thing you could be was a doctor.”
For college, Brown set his sights on Princeton. “It had nothing to do with education,” he says. Rather, Princeton men “were sophisticated. They smoked pipes; they had ascots.”
Princeton accepted him, but the school did not offer a scholarship, which put it out of range. In contrast, the University of Pennsylvania paid all of his expenses through a program sponsored by Proctor and Gamble. When Brown went to a dinner that honored the scholarship winners — two from each class — he met the other awardees. “The other seven guys are all about 6 feet 5 inches tall,” says Brown. “Here I was, this chubby little kid.” He told the dean of students, “I think you made a mistake. They’re all athletes.” The dean assured him that there had been no error. “You’re the most important one,” he responded. Proctor and Gamble did not want to use the funds to recruit athletes, and the recipients’ grades were feeble, so the company was getting suspicious. Penn needed someone who would raise the group’s GPA.
Whether analyzing circuits, cells, or patients, Brown defines problems and imagines solutions; he is shown here (third from right) making rounds in medical school.
Brown studied chemistry and “fell in with a group of newspaper writers and editors who were rebelling against something we called ‘in loco parentis.’ We believed that we were grown men and did not need parental substitutes who enforced things like curfews and segregated the women from the men, which had the effect of denying women an equal education,” says Brown. The staff raised a ruckus through their reporting, and the university administration tried to quash the rabble rousing. The brouhaha culminated in a self-organized protest by the newspaper staff against its own publication, conceived by Brown to gain TV coverage about the situation. He may have originally thought he wanted to live a smoking-jacketed existence, but some internal compass was pointing in a different direction. Convention confined his independent spirit.
Brown decided to stay at Penn for medical school because, by the time he was applying, his high school sweetheart, Alice Lapin, had begun college nearby. They had met during a summer on Long Island when she was 14 and he was 16. “Michael had a driver’s license,” she says. “I thought he was cute.” During medical school, they wed — and Brown also shot to the top of his class.

Brown and his wife, Alice, forged a marriage that has thrived for 59 years (1964).
The marriage has prospered, and Brown routinely expresses gratitude toward Alice. Among other things, she understands that his work compels him. In a TED talk on how to win a Nobel Prize in nine simple steps, he ends with homage to his wife. “None of the other steps matters if you make a mistake on this final step — the most important step of all,” he says: pick the right spouse. He attributes his success to two partnerships — one with Joe and one with Alice.
She shares his perspective. “A [professional] partnership only works if the family part works,” she says. Often “the family breaks up or the partnership breaks up.” People get frustrated with a mate who works too many hours or is not home to help with the kids or the house. “I just ignore that,” says Alice.
A winning beginning
United at MGH, the two superstar students quickly found common ground. Over midnight meals, they discussed the patients they had just admitted — not just what was wrong with them, but the underlying physiology of their ailments. “We had a deep interest in the fundamental science because it would make us better doctors,” says Brown.
During their second year, they started having a few nights off in a row and began playing duplicate bridge. This activity deepened their friendship, says Brown. “For one thing, I realized [Goldstein] has patience and doesn’t explode if I make a stupid mistake.”
Goldstein began recruiting Brown to UTSW. In the MGH emergency room, they would sit at the doctors’ station “and take out a paper and pencil and rank the UT Southwestern faculty against the MGH faculty,” says Brown. “Joe would tell me about this person [at UTSW] who knew more about kidney disease than anyone at MGH. By going through on a person-by-person basis, it was clear that Southwestern was better.”
Seldin came to MGH as a visiting professor and wowed Brown. “The house staff presented their most difficult patients, the ones they couldn’t figure out,” he says. “The way Seldin thought about [the clinical puzzles] was just so brilliant. He blew everyone away.”
Building up to cholesterol
Before the end of medical school, Brown and Goldstein had applied to the NIH Clinical Associate Training Program. This service replaced a military stint in Vietnam, and competition was fierce. Their stellar records earned them acceptance.
The NIH environment electrified them. In addition to performing clinical duties, they immersed themselves in their laboratory studies. Key leaders at the agency had been “smart enough to bring in basic scientists,” says Brown, so he and Goldstein benefited from exposure to this core group that focused on isolated aspects of physiology rather than disease per se.
A “great thing about being at the NIH in the 1960s was the large number of people who were doing original work,” says Goldstein. Over the next several decades, six of the NIH career investigators who were there at that time went on to receive Nobel prizes, and seven of Brown’s and Goldstein’s NIH peers earned this distinction later, at other institutions. “Many Jewish scientists who were really first rate ended up at the NIH because they couldn’t get jobs anywhere else,” says Goldstein. “And women. And husbands and wives.” By hiring talented individuals who had trouble securing university positions, the agency created a rich setting.
Goldstein joined Marshall Nirenberg’s lab, and within a few months, Nirenberg won the Nobel Prize for cracking the genetic code. Goldstein studied the last steps of protein synthesis and published three first-author papers and three coauthor papers during his two years in the lab. He not only generated a steady flow of high-profile results but also absorbed Nirenberg’s exacting rules for writing. Nirenberg drove home two other crucial lessons: the importance of attending to minute experimental detail and using pure components.
Brown’s initiation into NIH research was disappointing, but through a series of serendipitous events, he switched into the lab of premier enzymologist Earl Stadtman. There, Brown mastered techniques of protein purification, and he applied them to a particular enzyme, glutamine synthetase. In a single fateful all-nighter, his ingenuity and experimental acumen led him to the astounding realization that the same enzyme was turning glutamine synthetase on and off.
“Once you’ve made a really important finding and had that experience, you want to do it again and again,”
says Brown. “Not for external recognition. For internal reasons,” and also, in the case of Brown and Goldstein, for each other. “To this day, each of us is trying to impress the other one,” says Brown. “It’s not one-upmanship. It means a lot to me if, every once in a while, I make some suggestion and Joe says, ‘that’s a good idea.’ And vice versa.”
Like Goldstein, Brown learned how to do meticulous and robust science at the NIH. The scientists harnessed these precepts and carried them onward; decades later, they are still passing them along.
At the NIH Clinical Center, they saw patients with rare maladies that had stumped community doctors and other specialists. In January 1969, Goldstein did the initial medical evaluation of a seven-year old named Valerie Harrell who would dictate the course of his and Brown’s careers. The LDL blood cholesterol level of this girl soared eight times above normal.
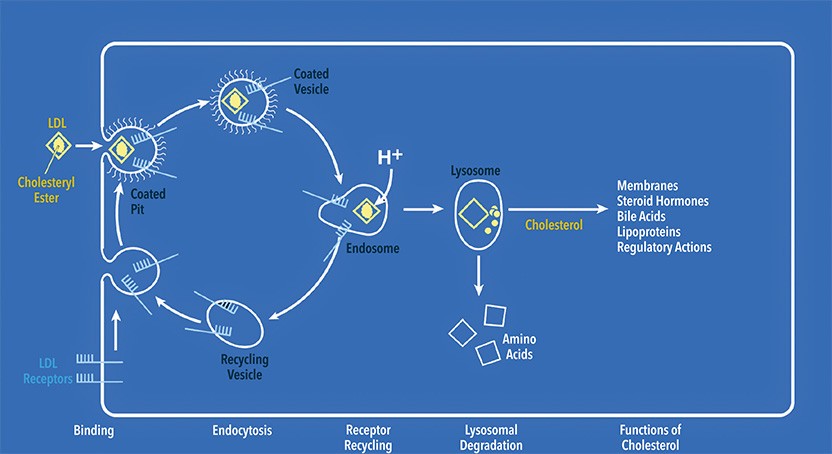
LDL particles bind to specific receptors located in specialized regions of the plasma membrane called coated pits. Coated pits with receptor-bound LDLs pinch off to form coated vesicles, which then fuse with a different type of vesicle called an endosome. There, LDL separates from its receptor, and the freed receptor returns to the plasma membrane. Endosomes containing receptor-free LDLs fuse with lysosomes, and the LDLs are degraded. Their cholesterol is released and enters the cytoplasm, where it performs numerous essential functions.
Cholesterol is an oily compound that dwells in the membranes that surround cells, where it confers fluidity and flexibility. The liver makes this essential substance, and humans also get it from food. Although we need cholesterol, it can cause trouble because excessive quantities clog arteries. This situation can lead to coronary heart disease, the top killer in the industrialized world. To transport cholesterol in the blood, the body packages it in particles, the most abundant of which is called low-density lipoprotein (LDL).
Valerie and her brother had familial hypercholesterolemia (FH), an inherited disorder that causes heart attacks in childhood. The siblings had received two damaged copies of a then unidentified gene, one from each parent, and thus suffered from the severe and uncommon form of the illness; it strikes about one in a million people. If this type of FH is not recognized and treated early, afflicted individuals typically die before adulthood. Those like Valerie’s parents, who carried only one defective copy of the gene, also have high cholesterol levels, but not nearly as extreme, and they do not get heart attacks until at least middle age.
Valerie defied the odds and is still alive 52 years later, thanks to a multitude of therapeutic interventions. She recalls the throng of doctors that appeared on rounds to learn about her condition. “Usually you would see them and then they would go on to other diseases down the hall,” she says. “But Dr. Goldstein came back. He was very inquisitive and wanted to know a lot of details.”
Goldstein remembers their encounter too. “Few people in the world had ever seen a 7-year-old girl who had a cholesterol that was around 800,” he says. “Without Valerie, we probably never would have studied FH.”

Fifty years after Goldstein first encountered Valerie Harrell (center), the little girl with FH at the National Institutes of Health Clinical Center, he and Brown re-met her and her mother, Treva Gray, at an event that was hosted by Alan Alda during the fourth episode of Soldiers of Science , which aired in December 2020 on Audible.
In the cafeteria, Goldstein told Brown about these children, and the burgeoning researchers wondered what genetic error might cause this massive cholesterol accumulation. They knew that cholesterol curbs the activity of a key enzyme in the biochemical pathway that manufactures it: when cholesterol is plentiful, output from the enzyme wanes; when cholesterol is scarce, it ramps up. In this way, cells tune cholesterol production to metabolic need. Heads bent together over lunch — a hamburger for Brown, a turkey sandwich for Goldstein — they hatched an idea. Perhaps, in people with FH, a flaw in the enzyme, HMG-CoA reductase, renders it insensitive to this feedback regulation. In this scenario, cells would keep making cholesterol even when swamped with the substance.
Per his agreement with Seldin, Goldstein went off to work with Arno Motulsky at the University of Washington Medical School in Seattle, with the goal of learning medical genetics. There he spearheaded a landmark study that unveiled genetic contributions of inherited lipid disorders to coronary heart disease.
At UW, Goldstein also learned how to extract connective tissue cells called fibroblasts from skin biopsies and then grow the cells in petri dishes for many generations, a technique that is standard now but that few investigators knew how to do at the time. He wanted to master this skill because he thought it might open the study of FH. Elizabeth Neufeld (1982 Lasker Award) used fibroblasts from two other inherited disorders to elucidate the underpinnings of these conditions. “We were right there when that was happening at NIH,” says Goldstein, and he recognized its implications. Her work established that some aspects of diseases that arise from faulty genes can be studied in cells grown outside the body. Not only that, the cells need not come from the parts of the body commonly associated with the malfunction. The promise of fibroblasts to help Goldstein probe FH was tantalizing. Although liver would, in principle, be the most obvious tissue to use, he hoped that fibroblasts would mimic liver cells, as skin samples are readily available.
In the meantime, Brown had acquiesced to Goldstein’s lobbying for UTSW, despite his and Alice’s deep Northeastern roots: when they went to the NIH, they thought it was the Deep South, he says. With trepidation, he turned down a job offer in San Francisco, “the most incredibly fabulous place,” says Alice. Alice was a trooper and agreed to give Dallas a try for a year.
Making it big in Texas
In 1971, Brown joined the internal medicine department at UTSW Medical School as a fellow in gastroenterology, with the goal of combining clinical work with laboratory research. When the Browns got to Dallas, “we looked for a Chinese restaurant,” says Mike. “There were no Chinese restaurants. There were no Italian restaurants. The culture shock in the beginning was pretty tough.”
Finding a dinner spot was not the only problem. By this time, Brown had committed himself to chasing down HMG-CoA reductase, the enzyme that he and Goldstein had pinpointed as the possible culprit in FH. Brown chose to work in the laboratory of an investigator who shared his interest in cholesterol, but their resonance did not extend to approach. The lab chief was exploring metabolism in rats, in large part through surgical studies. Having savored the precision of biochemistry, Brown wanted to work with enzymes, not splayed rodents.
Brown phoned Goldstein in Seattle and said, “How did you talk me into this? This is the worst place in the world.” He also contacted Boston. The head of the MGH gastroenterology unit told Brown he could return, but not until July, when the next medical training cycle would start. Goldstein prevailed upon Seldin to do something, says Brown. Seldin arranged for Brown to conduct independent work.

Luminary Donald Seldin with his protégés Goldstein and Brown, who followed in his footsteps to realize Seldin’s vision of transforming University of Texas South Western into a world-class medical institution (2017).
Brown told his mentor, Stadtman, about the project he had in mind, and the elder scientist was skeptical. Stadtman’s idol — Feodor Lynen, who discovered HMG-CoA reductase — had tried for years to purify the enzyme. The notion that this newbie would prevail seemed laughable, and Stadtman literally laughed.
Part of the challenge was that the enzyme resides in a cellular membrane, whose fatty nature makes it insoluble in reagents that are used to separate proteins from one another. Brown charged ahead even though no one had purified any membrane-bound enzyme at the time. Lynen had deemed this particular task impossible because he thought HMG-CoA reductase had to be attached to the membrane in order to perform its job. If so, no one could free it in a functional state.
“I thought it had to be done,” says Brown, because figuring out how a regulated enzyme worked posed “a tremendously interesting problem. I didn’t have anything to lose. If it didn’t work, I’d be a happy doctor.”
The turning point came when he realized that he was inadvertently inactivating the enzyme while centrifuging it. The problem was the cold at which the machine operates to avoid heating the rotor as it spins. In a spirit of amateur engineering that is hard to conceive for most biochemists, but easy to conceive for a former teenage radio tinkerer, Brown removed the control panel and disconnected the wires that ran the refrigeration system. In this way, he avoided debilitating the enzyme during the centrifugation step, separated its activity from the membrane, and laid the groundwork for its purification.
Things were getting exciting, he told Alice, and Joe was coming back. He floated the idea of staying for a couple more years. “I wasn’t moaning and groaning,” says Alice. “I had made some friends and I didn’t think it was forever.” As it turned out, the results were always “more and more exciting,” she says. Almost five decades later, the Browns are still in Dallas.
By the time Goldstein returned in 1972, Brown was flourishing. He had earned status within UTSW, which was crucial to their alliance because he did not want colleagues to view him as Goldstein’s sidekick. Still, they needed to hash out agreements about how they would work together. Goldstein was already established in the field, says Brown. “He was brilliant as a medical student at Southwestern. In Seattle, he published these brilliant papers about cholesterol and heart attacks. So this business of sharing credit was at my insistence. Otherwise it wouldn’t have worked.”
They would alternate authorship, they agreed, switching the senior spot with each publication. Regardless of who was invited to give an honorary talk, they would take turns accepting. Neither would claim an idea, thus avoiding “egotitis,” as Goldstein puts it. If one of them had a dazzling thought on Thursday, it probably came in part from something the other had said on Tuesday.

Brown and Goldstein have successfully tackled numerous scientific challenges in their five decades collaboration.
Initially, Brown and Goldstein planned to run independent labs and collaborate only on the FH project. They began to test their idea that people with FH carry a glitch in the gene for HMG-CoA reductase that foils cholesterol’s normal ability to muzzle the enzyme’s activity.
When the investigators added LDL to the broth in which normal cells were growing, enzyme activity dropped; when they removed it, enzyme activity rose. These results encouraged them. The feedback regulation system in fibroblasts seemed to be intact.
Next, they compared the behavior of cells from individuals with and without FH. Brown was out of town at a meeting, but Goldstein knew what technician Suzanna Dana was doing that day. After medicine rounds with students, he rushed to the lab, and Dana handed him the printout. He could hardly believe the numbers. HMG-CoA reductase activity blared 100-fold above normal in FH cells, a difference that biologists rarely see. “When you do, you know you’ve got something,” says Goldstein.
The observation held up. Furthermore, in contrast to normal cells, which halt production of cholesterol when LDL is available, FH cells ignore LDL. They crank out cholesterol even when awash with LDL.
In the meantime, Brown and Goldstein had discovered that their original hypothesis was incorrect: the FH glitch did not trace to HMG-CoA reductase after all. They postulated the existence of “a hitherto unidentified gene whose product is necessary” to subdue HMG-CoA reductase activity in response to LDL.

Whether zooming in on details or panning out to take in the big picture, Brown’s and Goldstein’s insights have produce novel views.
When the researchers added pure cholesterol rather than LDL to FH cells, it stymied HMG-CoA reductase just fine. This observation meant that the diseased cells contain everything they need to foil enzyme activity. Something was going awry as LDL-wrapped cholesterol was being transferred from the outside to the inside of the cell.
Additional experiments led Brown and Goldstein to propose that a molecule on the cell surface grabs LDL and that people with FH harbor defects in the gene that encodes this receptor. The idea met considerable resistance. When the pair submitted the results for publication, one reviewer wrote, “This is a most interesting paper and I wish I could recommend its acceptance without reservations… It is my considered opinion that publication of this paper with its incomplete observations would not serve medical science neither would it earn credit in the long run to its authors.” Many experts, says Goldstein, “couldn’t believe cholesterol could enter the cell in a specific way,” because conventional wisdom held that cholesterol “just melded into the membrane.”
Eventually, Brown and Goldstein established that the scheme they outlined was correct, and they illuminated the detailed mechanism by which cholesterol enters cells using LDL receptors and is then liberated for use inside. Furthermore, they showed that cells churn out additional LDL receptors when they need cholesterol. This system drains the artery-choking substance from the bloodstream. Because nonfunctional or absent LDL receptors cannot internalize LDL, people with FH cannot clear cholesterol, and it lodges in their arteries.
These and other findings delivered a rationale for the development of the cholesterol-lowering drugs called statins, a potent treatment for people with coronary heart disease. Work that began with a one-in-a-million child gave rise to therapy that has benefited millions of people worldwide. Brown and Goldstein’s revolutionary discoveries about the LDL receptor opened a floodgate, and we now know that the ability of receptors to pluck particular items for import from the external environment plays a key role in a multitude of physiological processes far beyond the field of cholesterol metabolism.

Prime Minister of the United Kingdom, Margaret Thatcher met with Brown and Goldstein to learn about cholesterol and statins.
While they were nailing down the receptor story, Brown and Goldstein merged their laboratories. “During the height of the excitement, we would work together constantly,” says Brown. “We were always planning the experiments for the next day” or crafting papers.
Writing was an intimately collaborative process. “It wasn’t like someone would write a draft and the other one would revise it,” says Brown. “Joe would be sitting there with a pencil and writing longhand on a pad and I would be spouting out sentences. Joe would start correcting single words and I would say, ‘let’s get the thoughts down and then go back and clean up the language,’ and Joe would say, ‘I don’t think we mean that word.’ The two of us would labor over every sentence.”
“In our heyday, we used to write 12 papers a year,” says Goldstein. “A typical paper would have 20 or 30 drafts. We don’t have any idea how we wrote papers before word processors.”
By the time Monty Krieger, a cell biologist now at the Massachusetts Institute of Technology, interviewed to do a postdoctoral fellowship in their lab in 1977, Brown and Goldstein could not keep up with their own output. Krieger noticed a pile of graphs on the floor in Goldstein’s office and asked what they were. Goldstein said they were experiments he and Brown had not yet had the time to write up. “Most scientists would give up a limb or a relative if all of their work amounted to that pile,” says Krieger, “and this was just what they didn’t have time to write up. I said to myself, ‘Oh my God, this is a rocket taking off.’”

Goldstein’s home town, population ~3000, broadcasts the glory of a renowned citizen. Cancer biologist Robert Weinberg points.

Brown’s high school honors its star scientific player with this sign on the football stadium.
That rocket has remained aloft and boldly on mission. Since the early results for which they won the 1985 Albert Lasker Basic Medical Research Award and the Nobel Prize, Brown and Goldstein have made leap after leap. They undertook a long and challenging quest to track down a family of proteins that govern cholesterol feedback, the sterol regulatory-element binding proteins, and untangled the novel strategy by which they work. This crusade has delivered an impact on far-ranging areas of biology including development, Alzheimer disease, and responsiveness to a range of stresses. Other advances have touched on myriad processes, from the genetic basis of a rare cause of blindness to a serious illness in which fat deposits in the liver and muscles to a mechanistic explanation for a devastating genetic condition, Niemann-Pick type C disease, which causes liver and neural degeneration in children.
Melding minds
Some people might make commitments about sharing recognition in a calm moment but struggle to honor it in shaky ones. Brown and Goldstein have managed to uphold the agreements, says Brown, because of “Joe’s incredible sense of security. You can’t insult him. He doesn’t take offense at anything. My wife tells me that I can get this tone in my voice that is threatening. Most people would react negatively if I used that tone. Joe just doesn’t hear it.”
Seldin contributed key elements too. He took an interest in both of them and supported their relationship. For instance, he grasped the consequence of equal treatment and promoted them in lock step, a practice that his successors followed. “The reason partnerships fall apart is that one or the other is considered to be the dominant person,” says Goldstein. “Our school has never allowed that to happen.”
Intellectual compatibility and mutual confidence are essential as well. “We’ve never disagreed on the big picture,” says Goldstein, “though we do disagree on details.” Furthermore, “we both have good taste for scientific problems.” Goldstein says he relishes working with a partner “who’s really smart and who I can trust. I don’t think we’ve ever told each other even a white lie.”
Brown notes that “credit became less of an issue when we began turning up totally unanticipated truths of nature. We were working day and night to solve these problems, so that kept us together. We gained great respect for each other’s intellect and talent and honesty.”
Brown, mischievous, includes Goldstein’s holiness in the list of fundamental relationship components. For a partnership to work, one person has to be a saint, he says, “and a saint I ain’t.” Goldstein teases back. “He likes to say that because it rhymes. Saint. Ain’t. Maybe I’m a saint compared to Mike, but I’m not a saint.”
Brown and Goldstein have managed to live and thrive by their ground rules for almost half a century. “It’s hard to explain how we work together,” says Goldstein.
“I think the key thing is a constant dialogue. Somehow all the nonsense gets weeded out along the way and one ends up with the best way to approach a problem.”
Others have witnessed this dynamic. They “come up with as many ideas as quickly as possible and then argue over them and, as quickly as they can, eliminate 95% of them so they’re left with the handful that are good and useful, the ones that survive their joint scrutiny,” says Robert Lefkowitz, a biochemist at Duke University. “Usually it goes something like this. Joe will put forward a hypothesis or idea. Mike, with no hesitation whatsoever, will say something like, ‘Joe, I’ve heard you come up with a lot of stupid ideas in your time, but this one takes the cake.’ And then he’ll demolish it. Joe, without getting the least bit angry or defensive will come back with something. Then they’ll go back and forth. It’s clear that they have no ego involved. The ideas that can withstand this withering cross-examination are worth pursuing.”
The partnership liberates Brown and Goldstein intellectually, says Helen Hobbs, a human geneticist at UTSW who did a postdoctoral fellowship in their lab. “If you have someone who you totally trust intellectually, you can make broad, big connections — even fanciful ones,” she says. “You can ask, ‘what is the most important thing that this could mean?’ because you have somebody who can edit or restrain you.”
They also provide reality crosschecks for each other, says Hobbs. “Sometimes when you present data, you want to see certain kinds of things that fit your hypothesis. There’s none of that. It’s so much less likely to happen because there are two of them.”
Their offices adjoin through a short corridor, and they can pop next door when they hear something that sounds interesting. “They expand each other’s worlds and the worlds of everyone around them,” Hobbs says.

The lab’s Japanese postdocs threw Goldstein a party to celebrate his 60th birthday, a magical event in Japanese culture, in which a person is said to experience a rebirth.
Compatible as they are, Brown and Goldstein are not identical — and people who have worked with them offer metaphors to describe the distinctions. Joe is the metronome and Mike is the rubato. They are speeding down a track; Mike pulls as fast as he can while Joe keeps the train on the rails. Joe is a firehose, spitting out ideas; Mike focuses them. Mike is thinking about how the data will solve the field theory of the universe and Joe brings him back to earth by asking about the magnesium concentration.
Brown and Goldstein caution against attempting to define differences in their thinking and style. People see only the outward face of their partnership, Goldstein observes, and most of their interactions occur privately. “We have a lot in common in the way we approach things.” Krieger sums up their union’s impact on their research: “Their relationship doesn’t influence their work; it is an integral part of their work.”
Legacy by example
The first December Krieger was in the lab, the postdoctoral fellows and technicians organized “a surprise and biting satirical skit” for the annual party, he says. Brown and Goldstein got wind of this plan. As the performance ended, “up leapt Mike and Joe,” Krieger recalls. “They said, ‘Thank you very much. You’ve had your turn. Now it’s ours.’” In their spoof, Goldstein interviewed potential postdocs, each of whom was played by Brown. “It was brilliant,” says Krieger. “They have a great sense of humor and timing,” and they nailed everyone’s quirks.

Goldstein (left) and Brown applied their prodigious talents to writing and performing skits as well as making scientific breakthroughs.
This familiarity enables them to tailor their mentoring. “They chose the perfect approach for me in that point of my development,” says Krieger, guiding him firmly at first, and eventually loosening their grip so he learned how to self direct in what was a new discipline for him. “I was not used to being in an apprentice/master relationship, but what they did could not have been better for me,” he says.
They’re tough, but it’s never personal, says Hobbs. When she presented results without the appropriate controls, “they would say, ‘just take that away. We don’t want to see it,’” she recalls. “I think that’s the right way to be. They weren’t maligning me as a person. They were just saying that what you just did is not the way to do it.”
They give her feedback on presentations too. “Joe will say to me: ‘Slide number 10, that’s a terrible slide.’” She appreciates the candor. “Who tells you these things? It’s hard to take at first, but then you realize that they want you to do your best science.”
Brown and Goldstein hold themselves as well as their charges to high standards. One summer, Krieger took on a medical student to supervise. When he told his mentors that he thought the work was ready to publish, they asked him to come in that weekend and bring all of the notebooks. “We went through, page by page, every experiment that was done,” he says. “They wanted to see everything. They did not want to see a graph of the data; they wanted to see the data. They wouldn’t feel comfortable reporting to anyone else — in a meeting, in a talk, in a paper — anything they weren’t completely certain about.”
Their scrupulousness extends to all aspects of their work, says Hobbs.
“Mike always said, ‘you can’t start down the slippery slope — throwing out one point because you think that rabbit was sick.’ There’s no tolerance for any kind of data manipulation.”
One time, Krieger recalls, he was toiling in the cold room — a walk-in refrigerator — alongside a labmate. She let out an expletive because she didn’t know how many times she had washed her samples and now she would have to start the entire procedure from scratch. Krieger offered sympathy; she had invested a lot of time, and the physical discomfort was significant. “Better to be cold than wrong,” she said, according to Krieger. “That’s always stuck with me,” he says. “Never ever be reluctant to do everything you have to do to make sure your work is reproducible and reliable. That comes from the environment they established.”

Brown-Goldstein lab postdoc reunion, held in Monterey, California. The photo includes Helen Hobbs, Thomas Südhof, Xiaodong Wang, Monty Krieger, David Russell, and many post-docs who came from Japan, China, and Europe (2015).
Indeed, Brown and Goldstein boast an extraordinary mentorship record that few scientists can claim. Five of their postdoctoral fellows or students have been elected to the National Academy of Sciences and two to the National Academy of Medicine. Ulrike Beisiegel, a former postdoc, was elected president of the University of Göttingen, Helen Hobbs won a Breakthrough Prize, and Thomas Südhof earned a Nobel.
For Brown and Goldstein, inquisitiveness rather than expediency leads the way — to an unusual degree. David Russell, a molecular biologist at UTSW, recalls a collaboration that illustrates this inclination. At one point, the team was ready to submit some results for publication that were correct, “but didn’t provide a lot of insight,” Russell says. “On Friday afternoon, we had finished the 30th draft of the paper, and it was all set to be put in a first-class, green-striped envelope to go out Monday.”
That did not happen. Saturday morning, Brown had an idea; maybe they had missed a far-fetched but possible explanation. A lively argument ensued about what the data could mean, says Russell, and eventually the researchers convinced themselves to do a few more experiments, which generated “an incredibly important observation.” Most people would have said, “This is done. Let’s move on to the next project,” he says. “Thirty years later, I remember that argument like it was yesterday — and how the outcome reflects their personalities and the consequences. They always get to the heart of the matter.”
Brown’s and Goldstein’s imaginative flair sparkles in multiple arenas. Every year, for instance, Goldstein, as chair of the Lasker Medical Research Awards Jury, makes unorthodox connections between art and science during his hallmark lectures at the Awards luncheon. “He puts together two things that you’d think would have nothing to do with each other,” says Brown.
Goldstein, now a modern-art aficionado and collector, took “a typical art history course in college,” he says. His interest took off when invitations from European institutions began streaming and he started tucking in museum visits. Goldstein enjoys all forms of art, but “if you’ve seen a couple of Rubens or Rembrandts, you’ve pretty much seen them all,” he says. “You have to acquire a taste for modern art. Some people hate it, but there’s a certain beauty in it.” Goldstein is a devotee of Ellsworth Kelly. “Every painting I’ve ever seen of his is a different shape and in different colors. And he did this for 50 years. The artists I like do something really creative over a long career rather than a single piece.”
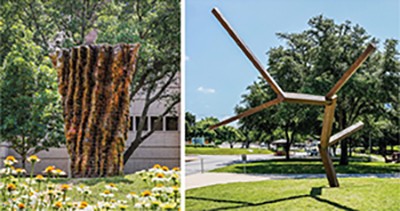
Goldstein donated two sculptures to the University of Texas Southwestern: “Dumna” by Ursula Von Rydingsvard (left); “Untitled” by Joel Shapiro (right).
Goldstein commissioned two sculptures for the UTSW medical school courtyard. “It was landscaped really well, but there was no art,” he says. Many individuals emailed him about the towering bronze pieces. “People who had never been to museums or seen art before really reacted to it. That was gratifying.”
Numerous institutions have attempted to lure Brown and Goldstein from the lab, but additional prestige and power have never unhitched them from the experimental science that energizes them. After they won the Nobel Prize, they asked themselves what they most liked to do, and the answer was clear, says Brown: come to the lab in the morning and encounter a graduate student or postdoc who is bubbling about new results.
“There are so many temptations, especially when so many doors are open to you,” says Xiaodong Wang, a biochemist at the National Institute of Biological Sciences, Beijing, who did a postdoctoral fellowship with the team. “At that young age, to have that kind of perception and discipline, I think, is a most remarkable thing.”
Goldstein has no second thoughts. “We have the best of all worlds,” he says. “If you become the head of something, you have to spend all your time doing that if you want to be successful at it. We can be on boards and advisory committees, and I think that’s more fun.”
Although they are far more interested in doing research than in amassing accolades for their discoveries, Brown and Goldstein delight in the celebratory atmosphere and the conversations that spring up at the festivities. At the Nobel Prize banquet, their seats faced each other at the table of honor that stretched across the courtyard, and they savored the timbale d’écrevisses before it was time to speak. Between courses, a trumpet announced each new prize category to the 1300 gowned and tuxedoed guests. As Brown listened to the other laureates, he realized that the Swedish woman next to him, who had attended many previous Nobel banquets, was dozing.
He stood up and leaned over the table to catch Goldstein’s attention. “You heard those two speeches,” Brown said. “They’re exactly the same as ours. I’m going to tell a joke.” Goldstein objected on the grounds of impropriety.
Brown pushed.
Goldstein acquiesced as long as the secretary of the Nobel committee promised to print the original text.
When it was their turn, the twin stars of medicine strode up the grand stairway to the podium and Goldstein delivered the eloquent remarks they had prepared. Brown tore up his notes and replaced his portion with a joke that pays tribute to Alice.
That event spotlights attributes that characterize each member of the scientific duo and their amalgamation: Brown’s irreverent streak, Goldstein’s ability to think on the fly, transparent communication, flexibility and courage to shake things up on both of their parts, and overwhelming mutual respect.
Looking back, Brown says he is surprised at his boldness, “but to me, the work was important to take seriously, not the prize.”
By Evelyn Strauss

