On Christmas Day 1984, Carol Greider walked out of a darkroom at the University of California, Berkeley, with a piece of x-ray film that captured the results of her latest experiment. A series of evenly spaced dark bands, which indicated DNA pieces of specific sizes, climbed the film like steps in a staircase. Then a 23-year-old graduate student in the lab of biochemist Elizabeth Blackburn, Greider was hunting for evidence of an enzyme that could lengthen telomeres, the protective caps at the ends of chromosomes. She and Blackburn suspected that the enzyme existed, but no one had detected evidence of it.

Greider is performing experiments as a graduate student in Elizabeth Blackburn’s laboratory at the University of California, Berkeley circa 1985.
Courtesy of Cold Spring Harbor Laboratory Archives, NY.
The repeating pattern on the film “was exactly what I expected to see” if the enzyme, now known as telomerase, was real, Greider says. She was elated but still harbored doubts. “I have an innate skepticism and wanted to be sure about what I saw.” But within a year she and Blackburn had confirmed the result.

Elizabeth Blackburn (left), Greider, and Jack Szostak holding the winged victory statuette at the 2006 Lasker Awards Ceremony
Discovering telomerase was the first of several landmark findings that eventually earned Greider the 2006 Albert Lasker Award for Basic Medical Research and the 2009 Nobel Prize in physiology or medicine. She shared both honors with Blackburn and another pioneering telomere researcher, molecular biologist Jack Szostak of Harvard University. When Greider began her studies, scientists knew little about telomeres. Her findings helped show that the structures are crucial for maintaining chromosomal integrity and that their deterioration can lead to disease. “Carol is one of the giants in the telomere field,” says Vicki Lundblad, a molecular biologist at the Salk Institute for Biological Studies. “Her body of work has been pivotal.”
Reversing Dyslexia
People have mistakenly assumed that Greider was working on Christmas in 1984 because she was a “nose-to-the-grindstone person,” she says. Her presence in the lab was more a reflection of her family situation. She hadn’t returned home for the holiday because she was estranged from her father. “I didn’t have anywhere to go.” So she went to the lab to check on the experiment she had started a week before.
Her family problems dated back to childhood. Born in San Diego in 1961, Greider describes her upbringing as “fairly chaotic.” By the time she was four, her family had moved across the country twice as her father changed academic jobs. When Greider was six, her mother died, and her alcoholic father struggled to raise her. He eventually married a woman who was jealous of Greider and abusive to her. “It was a difficult time,” she recalls.
Because of that turmoil, she learned to shut out distractions and concentrate on whatever goal she was pursuing. As Greider recalls, “focusing on work and school would allow me to ignore what was going on around me.”
That ability would prove invaluable throughout her life, starting in grade school. At first she struggled because of dyslexia, although no one at the time recognized the problem. Greider’s grade school placed her in remedial spelling classes and required her to work with a spelling tutor. “As a kid, I thought of myself as stupid because I needed remedial help,” she remembers. By junior high, however, she excelled in her classes, an improvement she ascribed to “my perseverance and love of reading.”
Greider says her upbringing instilled another key trait that helped her succeed in science: independence. From 1st to 12th grade, she and her older brother, Mark, walked or rode their bikes to school instead of getting a ride. When she was 10, her family lived for a year in Heidelberg, Germany, where her father was taking a sabbatical. Greider explored the city on her own, using public transportation. Children today are more sheltered, but she says she tried to foster the same independence in her own two children, allowing her daughter to ride a bike to school in fourth grade. “My neighbors thought I was a bad mom.”
Both her parents had PhDs—her father was a nuclear physicist and her mother, a biologist—but Greider was not a junior scientist. “I didn’t have a chemistry set as a kid.” She decided to major in biology only after having enjoyed her high school biology class. “I thought, ‘This could be fun.’”
Playing in the Lab
When Greider started as an undergraduate at the University of California, Santa Barbara, she wanted to study marine biology. But to explore her options, she tried working in several biology labs. Marine biology research left her cold, she found, because it entailed only observation and statistical analysis. Biochemistry, however, was congenial because she could manipulate the systems she studied. “Once I landed in a biochemistry lab, I thought, ‘This is what my mind likes doing,’” she recalls. “I want to make changes and see the effects, not just observe.”
Greider continued to work in biochemistry and molecular biology labs throughout her undergraduate years. Her dyslexia nearly derailed her plans for graduate school, however. Because of her poor scores on the admission test, six of the eight universities she applied to rejected her outright. But two offered her interviews, including Berkeley. During Greider’s visit there she met Blackburn, whose enthusiasm captivated her. “She is very charismatic,” Greider says. Blackburn’s research on telomeres “piqued my interest because it was something very new.” She decided she wanted to join Blackburn’s lab.
Telomeres offered plenty of mysteries for Greider to investigate. Composed of proteins and repeated sequences of DNA, the structures form the tips of chromosomes. In 1984, researchers knew that the standard DNA-copying enzymes couldn’t completely duplicate the DNA at the ends of chromosomes, meaning that telomeres should shrink every time a cell divides. Yet scientists had found that in some organisms, telomeres elongate with every generation. One possible explanation for the discrepancy was that cells deploy an enzyme to extend the telomere DNA. The trick was to find it.
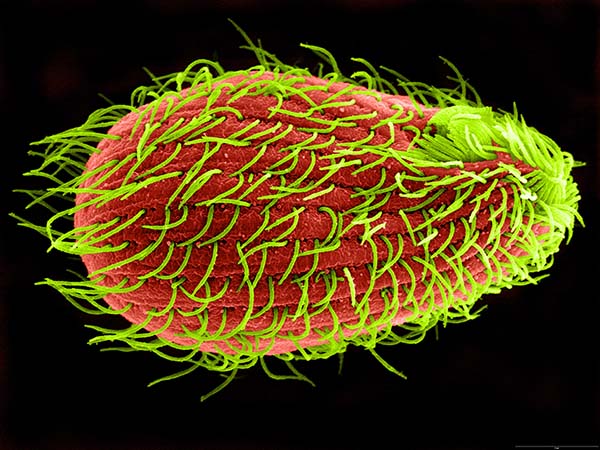
Color enhanced scanning electron micrograph of Tetrahymena thermophila, a freshwater ciliate approximately 50 micrometers in length and covered with 700-800 cilia (magnification X 2000)
Courtesy of Aaron J. Bell / Science Source
To improve the odds, Greider and Blackburn enlisted a single-celled pond organism called Tetrahymena thermophila that sports a whopping 40,000 telomeres—most human cells have 92. The pair assumed that Tetrahymena cells needed large amounts of the enzyme to maintain all those telomeres. Scientists hadn’t devised a standard procedure to identify an enzyme like that, so Greider improvised several experimental approaches. “We changed the reaction conditions, the substrates, and even method of detection,” she wrote. But the results never showed clear evidence for the enzyme.
In December 1984, after Greider had been working on the project for more than seven months, she finally succeeded. She had been adding lengths of DNA that resembled telomeres to the contents of Tetrahymena cells and then testing whether the telomeres grew. This time, she used a smaller, synthetic DNA segment. The approach yielded 1,000 times more DNA ends for the enzyme to interact with. She and Blackburn initially dubbed their enzyme “telomere terminal transferase,” but one of Greider’s friends jokingly suggested shortening it to “telomerase.” The name stuck.
Still, the researchers weren’t ready to declare that they had discovered an enzyme. “I thought, ‘Can this be true?’” Greider recalls. “It could be something else fooling us.” For example, one of the known DNA-copying enzymes might have lengthened the sequence. To rule out other explanations, Greider spent nearly a year running further experiments. Once she got results that confirmed the discovery, Greider says, she played Bruce Springsteen’s Born in the USA album and “danced around the house.”
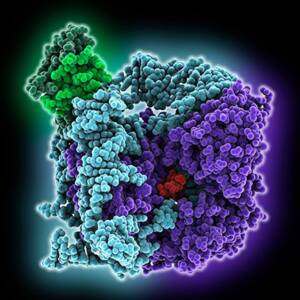
Molecular model of telomerase catalytic core: reverse transcriptase (violet), telomerase RNA (cyan), short telomeric repeat substrate (red), H2B type histone (light green), histone H2A (dark green)
Courtesy of LAGUNA DESIGN / Science Source
She may have celebrated, but other scientists mostly ignored the result, which Greider and Blackburn published in Cell, a top journal in the field, in December 1985. One reason for their indifference, Greider says, is that the study involved “a funny little organism,” so other scientists thought the findings weren’t relevant for their research. But they soon began to pay attention when other researchers showed that yeast and human chromosomes also carry telomeres with repeated sequences, she says.
Greider and Blackburn next wanted to probe how the enzyme works. One question they tried to answer was how telomerase could affix specific, repeated sequences to the ends of chromosomes. Most enzymes are proteins, but Greider and Blackburn speculated that telomerase was a dual molecule that also contained RNA. The nucleotides in the RNA portion could serve as a template for extending the telomere DNA. Greider tested the idea by adding an RNA-splicing enzyme to their experimental mixtures. The RNA-destroying enzyme stifled telomerase’s activity, suggesting that the hypothesis was correct. Greider then spent several months trying to isolate the enzyme’s RNA component, but it eluded her. Nevertheless, she and Blackburn thought they knew enough about telomerase to publish a profile of the enzyme in 1987, again in Cell.
Back East
By 1987, Greider had finished her PhD research and was looking for a job as a postdoctoral scientist. A professor at the Cold Spring Harbor Laboratory (CSHL) in New York, a leading institution for molecular biology research, invited her to work in his lab, which studied how cells copy DNA. However, CSHL also offered her the opportunity to run her own lab, which would allow her to continue working on telomeres. “I did not know there was such a position,” Greider recalls. “I was caught off guard” by the offer and initially turned the job down. But she quickly reconsidered and accepted it.

Carol Greider is with her laboratory group at Cold Spring Harbor Laboratory, 1993. Top: Carol Greider; Middle row, left to right: Lin Mantell, Kathleen Collins, Chantal Autexier; Bottom row, left to right: Ariel Avilion , Lea Harrington, Matthias Scherring, Stephanie Goldsmith.
Courtesy of Cold Spring Harbor Laboratory Archives, NY.
At CSHL, where she worked for 10 years, Greider finally nabbed the elusive RNA piece of the enzyme and began delving deeper into the functions of telomeres and telomerase. In the early 1960s, researchers had noticed that human cells growing in culture could divide only a certain number of times. What set that limit was unclear. A 1990 study by Greider and colleagues suggested for the first time that telomeres were responsible. The scientists showed that the telomeres of human skin cells growing in the lab shrank every time the cells divided. “It was a groundbreaking paper,” Lundblad says. Further research revealed that cells whose telomeres become very short eventually stop dividing or die.
In 1997, Greider moved to the Johns Hopkins University School of Medicine. She was part of a team that generated and tested mice genetically engineered to lack telomerase. The rodents were initially healthy, even in old age, the researchers revealed in 1998. But mice have naturally long telomeres. When the team interbred the telomerase-lacking animals over several generations, the rodents’ telomeres became shorter and shorter and health effects began to appear. The mice were less fertile, in part because males produced fewer sperm. Both sexes’ ability to generate new blood cells also declined.

From left to right: Greider, Titia De Lange, and Elizabeth Blackburn at the Telomeres and Telomerase Meeting, 2001
Courtesy of Cold Spring Harbor Laboratory Archives, NY.
The Toll of Short Telomeres
Some researchers have postulated that telomere shortening drives aging. However, Greider says, aging involves many changes in how cells and organs function, and telomeres alone do not dictate life span. Still, her research revealed that the structures do have a major effect on our health. Cancer is one example. Telomere shortening is a disaster for cancer cells because it can stop them from dividing. Most cells in the body don’t make telomerase. Once Greider identified the enzyme, researchers found that many cancer cells start producing telomerase to prevent their telomeres from becoming too short.
Greider’s research linked telomere shortening to other diseases. For instance, she, her colleague Mary Armanios, and their coworkers found that patients with a fatal lung condition called idiopathic pulmonary fibrosis carry mutations in the genes for the protein or RNA portions of telomerase. As a result, the patients’ telomeres shorten with each generation and may eventually trigger lung problems.
One reason that Greider has been so influential is that “she is an incredibly strong experimentalist,” Lundblad says. Greider combines that trait with a focus on experiments that continually reveal insights about how telomere length is controlled in cells, Lundblad adds. Greider’s exacting standards for research are another reason she has made such an impact, says biochemist Lea Harrington of the University of Toronto, Greider’s first graduate student. “She set the bar for many of us as to what science should be.” Harrington says that she and Greider’s other graduate students and postdoctoral researchers have now passed that way of thinking on to a new generation of scientists. “They essentially learned from Carol because we learned from Carol.”
Greider has been a role model for women in telomere research and other areas of science—she and Blackburn were the first two women to win the Nobel Prize in the same year. Greider also has spoken out about sexual harassment, which she says drives many women from scientific careers. She served on a panel in 2019 that drew up recommendations for how the National Institutes of Health should deal with sexual harassment by researchers who receive or apply for agency funds. Greider’s efforts not only helped draw more women into science but also have benefited the rest of the field, Harrington says. “She provides that leadership for everyone.”

Back in her native California, Greider is learning to surf.
Courtesy of Christopher Michel
After spending 22 years at Johns Hopkins, Greider now investigates telomeres and their role in disease at the University of California, Santa Cruz, where she became a professor in 2020 after another transcontinental move. During her last 17 years at Johns Hopkins, she was head of her department. But the administrative demands became more and more burdensome, cutting into the time she could devote to research and students. Now, “I have traded administration for teaching,” which she enjoys. And Greider has more time to pursue the lab work she loves. For her, research is “play,” she says. “It is solving a puzzle.”
By Mitchell Leslie

