In the mid-1970s, Christiane Nüsslein-Volhard was struggling. Two years after finishing her PhD in molecular biology, she was working as a postdoctoral researcher in a lab she longed to leave. The new European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, had just turned her down for a job because she didn’t have enough experience working with the organism she wanted to study, the fruit fly Drosophila melanogaster. Worried she would soon be unemployed, Nüsslein-Volhard wrote to several scientists and “begged them to give me a job,” she recalled in a 2015 interview.
Then Nüsslein-Volhard got a break. EMBL decided to hire her after all, but she would have to share a small lab with another scientist—who turned out to be her friend and scientific sounding board Eric Wieschaus. The pair went on to carry out a landmark study, known as the Heidelberg screen, that for the first time pinpointed many of the genes that choreograph embryonic development.

Nüsslein-Volhard at a Development and Genetics Meeting (1996)
Courtesy of Cold Spring Harbor Laboratory Archives, New York
“It’s the best project I’ve done in my life,” Nüsslein-Volhard has remarked. Other scientists agree, saying that the research transformed the understanding of development. “It has to be one of the most successful enterprises in modern biology,” says Michael Levine, a developmental biologist at Princeton University in New Jersey who was not involved in the work. The genes the researchers identified are “a who’s who of the movers and shakers in developmental biology,” he says. As scientists later discovered, many of the same genes also shape embryos in other animals, including humans, and some are culprits in diseases such as cancer. For that project and later research, Nüsslein-Volhard earned a share of the 1991 Albert Lasker Basic Medical Research Award and the 1995 Nobel Prize in physiology or medicine.
Developing an Interest
Nüsslein-Volhard followed a circuitous route to the pinnacle of developmental biology. She was born in Magdeburg, Germany, in 1942 and grew up in Frankfurt, where her father worked as an architect. Her parents and four siblings were artistic rather than scientific, and she painted and played music with them. But she also was captivated by living things—birds, the plants in the family’s garden, the animals at a nearby farm she visited during school vacations. By age 12 she had decided to become a biologist.

Nüsslein-Volhard takes notes at a meeting of European fly researchers around 1980.
Credit: Ruth Lehmann
As a child, Nüsslein-Volhard has written, she often became “intensely interested in things, obsessed by ideas and projects.” However, her enthusiasm for certain subjects didn’t always translate into classroom performance. Recognizing her promise, her high school teachers concluded in their final evaluations—which she quoted in her Nobel Prize autobiography—that “she is gifted above average, has a critical and qualified judgment, and the talent for independent scientific work.” They also noted that she often didn’t apply herself and “with her strong display of self-will she can be decidedly lazy in some topics.” Nüsslein-Volhard admitted that because of her failure to study hard she earned a middling score on her high school final exam and almost failed the English section.
Over the next decade, she struggled to find an area of study that would hold her interest. At Frankfurt University, which she entered in 1962, the biology courses were mostly uninspiring, so after two years she left for a new biochemistry program at the University of Tübingen. Although the curriculum there was more to her liking, it offered too little biology. She finished “as usual for me, with rather mediocre grades because I had not always paid attention, and often had lost interest,” Nüsslein-Volhard wrote in her autobiography. Her next stop was the PhD program at what is now the Max Planck Institute for Biology Tübingen, where she devised a new method for purifying RNA polymerase, a key enzyme in protein synthesis. She also delved into the enzyme’s interactions with DNA. The results were important enough to yield scientific papers, but Nüsslein-Volhard acknowledged that she was bored with the work by the time she finished it.
Fixating on Flies
Other scientists in Tübingen were performing research that caught her imagination, inspiring her to begin investigating how genes orchestrate embryonic development. Fruit flies, she realized, were ideal organisms for that research because scientists already knew a lot about their genetics.
As with other species, including humans, the embryos of flies undergo a dramatic transformation as they develop. A freshly laid fruit fly egg is a nondescript globule of cytoplasm smaller than a poppy seed. By the time it hatches about a day later, it has morphed into a miniature maggot with distinctive front and rear ends, a nervous system, mouthparts for munching on yeast, and an organization of body segments similar to that of the adult fly. Researchers knew little then about how genes managed developmental changes in flies, let alone in other animals. “That was good. You could really dig in and find something,” Nüsslein-Volhard said in 2018.
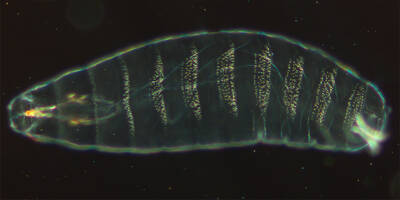
Drosophila melanogaster embryo
She began probing fly development during postdoctoral stints at the University of Basel in Switzerland and the University of Freiburg in Germany. Nüsslein-Volhard became obsessed with the insects. “I dreamt of flies,” she told an interviewer in 2015. “When I closed my eyes, I saw fly embryos.”
Her research on the insects took off when she started at EMBL in 1978. She and Wieschaus had to not only share the same small lab but also split the services of the same lab technician, Hildegard Kluding. So they decided that working on the same project made sense. The Heidelberg screen that they carried out over the next two years involved feeding male flies a DNA-damaging chemical that caused mutations in different genes. The pair crossed the flies’ offspring for several generations so that a relatively large proportion of the embryos carried altered genes and suffered harmful effects. Nüsslein-Volhard and Wieschaus then collected the embryos and checked for aberrant anatomy.
The research showcased several of Nüsslein-Volhard’s scientific strengths. It required a massive amount of effort. In total, the scientists reared nearly 27,000 families of flies. They spent hour after hour staring through a dissecting microscope outfitted with two eyepieces, which allowed both of them to simultaneously inspect embryos for abnormalities. Finishing the work required plenty of the self-will that Nüsslein-Volhard’s high school teachers had remarked on. “She is the most driven person,” says Ruth Lehmann, director of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and Nüsslein-Volhard’s former graduate student.

Nüsslein-Volhard at “Fliegenstall,” Max-Planck-Institut, Tuebingen (1992)
Image Courtesy of Archives of the Max Planck Society, Berlin
Another of Nüsslein-Volhard’s attributes, creativity, also was on display in her work on the screen, which “was quite an original sort of science,” says developmental biologist Peter Lawrence of Cambridge University in the United Kingdom. No other developmental biologist had tried anything like it. Many scientific discoveries have been made possible by the invention of a new technology, but that wasn’t the case for the screen, Levine says. The study “could have been done 50 or 60 years before, but nobody thought of it.”
The researchers also broke intellectual ground when they tallied the mutations’ impact on fly embryos. Other scientists who tried to understand how genes shape fly development focused on how mutations affected adult flies’ anatomy. That decision “was really important for the success” of the screen, Lehmann says, because the embryo is simpler than the adult fly and easier to understand. Another ingenious choice the pair made was to search for specific defects on the surfaces of dead embryos, Levine says. The corresponding fatal mutations resided within genes crucial for controlling development.
Nüsslein-Volhard’s artistic ability also proved invaluable, Lawrence says. A fly embryo is shrink-wrapped in a protective layer called the cuticle. To discern developmental flaws, the researchers had to parse the cuticle, looking for often subtle changes, such as alterations in the pattern of bristles on the embryo’s back or the arrangement of toothlike projections on its belly. With her artist’s eye, Nüsslein-Volhard was attuned to such changes, Lawrence says. Wieschaus also came from an artistic background, and the two stayed alert during long sessions at the microscope by competing to see who could spot a developmental change first. “It kept you awake,” Nüsslein-Volhard remembered.
Some scientists had worried that the screen wouldn’t be helpful because it would turn up too many genes. But after Nüsslein-Volhard and Wieschaus finished poring over embryos and cataloging the developmental defects, they identified a mere 120 embryonic genes that shape the cuticle and segmentation pattern. In 1980 they spelled out the effects of 15 of the genes in a paper in the journal Nature.
“I drooled when I read that paper. It was a treasure trove of information,”
says Levine, who was then finishing his PhD.
Lawrence says that the results “had an enormous effect on the field.” Scientists raced to isolate the genes and determine how they mold the fly embryo. And as researchers later discovered, counterparts of those genes sculpt development in many animals and go awry in diseases such as cancer. For instance, one gene implicated in the Heidelberg screen was wingless. It belongs to the Wnt family, whose developmental roles in other animals include differentiating the embryo’s front end from its rear end and its back from its belly. In addition, mutations of genes in the Wnt pathway occur in about 90% of colon cancers and in other types of tumors. Researchers are testing a variety of potential treatments that target the proteins.
In 1981 Nüsslein-Volhard returned to the Max Planck Institute in Tübingen, where she spent the rest of her career. She continued to hunt for genes crucial for fruit fly embryonic development. Her previous work had focused on genes active in the embryo. But the mother fly’s genes also help organize the embryo’s development, and her next project entailed identifying those maternal genes—a harder task because it required more rounds of fly breeding. However, Nüsslein-Volhard, who now headed her own research group, could count on more help, including from Lehmann. Nüsslein-Volhard was good at selecting a team of researchers who meshed, Lehmann says. “If people are going to spend most of their hours together, it’s good if they have the same drive and are interesting.” Over the next several years, the scientists uncovered another 40 development-managing genes.
Taking the Plunge on Zebrafish
Nüsslein-Volhard won the Lasker Award in 1991 largely for her work on fruit flies. But even before that research wound down, Nüsslein-Volhard was looking to move on and begin analyzing how genes coordinate development in vertebrates. “She had the vision to recognize that the field needed a vertebrate model comparable to Drosophila,” says Mary Mullins, a developmental biologist at the University of Pennsylvania who was Nüsslein-Volhard’s postdoc in the early 1990s. For her new study organism, Nüsslein-Volhard settled on zebrafish, which scientists had recently begun using in research on development. As she told an interviewer in 2017, she began to dream about fish instead of flies.

Nüsslein-Volhard switched model organism in the mid 1990s and chose the zebrafish.
Screening the fish “was a major operation,” Mullins says. Printed in a small font size, the schedule for the project was a meter and a half long, Mullins recalls. To house the 7,000 aquaria the researchers would need, Nüsslein-Volhard’s institute had to construct a new building. Nüsslein-Volhard, who has said that she loves working with her hands, also dove into the details of the project. For example, she helped design the fish’s aquaria so that they were self-cleaning—unusual for the time. As a result, technicians wouldn’t have to remove the fish to scrub their tanks, reducing the chances of mix-ups that could confuse the results, Mullins says. For scientists performing lab research, Lehmann says, “The practical aspects are just as important as the theoretical aspects. Nüsslein-Volhard excels at both.”
Like the fly screens, the zebrafish project uncovered a trove of crucial genes that govern embryonic growth. In 1995, Nüsslein-Volhard’s lab and groups at other institutions published 37 papers that identified 1,200 development-altering mutations in the fish. Researchers are still working to understand how those mutations redirect the fish’s development and their role in diseases.

Nüsslein-Volhard with her long time friend and collaborator Eric Wieschaus (2010s)
Courtesy of Juan Modolell
Nüsslein-Volhard has made an impact on science in other ways. Unlike researchers who jealously guard their discoveries, Nüsslein-Volhard gave many of hers away, Levine says. For example, when he had just started his own lab at Columbia University in New York City in the mid-1980s, he contacted her about collaborating to investigate a gene, known as even-skipped, that she had found in her screens. Instead, she sent him a box containing tubes of flies that carried mutations in the gene along with detailed instructions about how to raise and study the insects. Levine had no previous connection to her, and she had no reason to help him, he says. But her generosity “set me up for my whole career.”
She also has worked to bring more women into science. Nüsslein-Volhard was a pioneer in that regard. She was the first (and remains the only) German woman to win a Nobel Prize in science. At the Max Planck Institute she became a director, a supervisor responsible for overseeing the research of multiple labs. Only one other woman back then had achieved that distinction. Nüsslein-Volhard was married briefly in the 1960s but never had children. To help women who do, in 2004 she used some of her Nobel Prize money to launch a new foundation. It provides grants of €500 per month to help woman graduate students or postdocs pay for housekeeping or childcare, allowing them to devote more time to science.
Nüsslein-Volhard retired in 2014 but continues to ponder the role of genes in development. Her latest passion, the subject of a book she wrote in 2019, unites her artistic and scientific sides. She is exploring how genes that shape development produce beauty, such as the striking pigmentation patterns on zebrafish. Researchers usually eschew discussing whether animals such as zebrafish are attractive, she has said, because the judgment is subjective. But as she told an audience in 2018, she is intrigued to find out “first why they are pretty, and second how do they get pretty?”
By Mitchell Leslie

