To walk a mile in Paul Nurse’s shoes, you might do well to head out into nature. Growing up in London, Nurse took long walks through the park to primary school, and the plants and animals he observed along the way first sparked his enthusiasm for science. Years later, as a post-doc at the University of Edinburgh, it was this same attention to the natural world that spurred Nurse to study some smaller-than-expected yeast cells he noticed under the microscope, leading to his first great insight into genes that control the cell cycle. As Nurse said in an interview several years ago,
“That’s how I view a lot of biology — you have to follow where nature takes you.”

Nurse as a postdoctoral fellow is pictured here with Pierre Thuriaux. The two worked together to show that cdc2 controlled the onset of cell division in fission yeast.
Throughout his career, Nurse has been following his research findings, along with funding and job opportunities and the wishes of his wife and two daughters, all over the United Kingdom. Those stunted yeast cells, which Nurse dubbed “wee” in homage to his Scottish then-home, turned out to be rushing through the cell cycle and dividing faster than normal because of a defect in a gene he called wee1. This gene normally puts the brakes on the linchpin cell cycle regulator in fission yeast, called cdc2. The next step was to clone cdc2 and other cell cycle-control genes, so Nurse moved south to the University of Sussex in Brighton, England, in the early 1980s, to learn the latest molecular genetics techniques. Several years later, he got a position at the Imperial Cancer Research Fund (ICRF), now the Francis Crick Institute, in London, where his group was able to find a virtually identical gene, called CDC2, in humans, paving the way for the discovery of a family of related genes that are now possible targets for cancer treatments.

Nurse spent his early postdoctoral research years in the lab of Urs Leupold, who was one of the first researchers to use fission yeast as a model organism. Nurse is pictured here with his lab mate and notable fission yeast researcher, Peter Munz.
But it would be misleading to suggest that Nurse’s path in science has been a walk in the park, as facile as his boyhood strolls to school. In reality, Nurse says he has stumbled through many failures. He felt inadequate in secondary school because his classmates came from families with more money and education, whereas Nurse’s father was a factory mechanic, his mother a cleaner, and no one in his family went to school past age 15. He barely got into college because he could not manage to pass a basic French exam, flunking it six times and eventually winding up at University of Birmingham only because a professor there noticed his otherwise strong application and made an exception.
Nurse even faced sizable hurdles in science, which was normally his strong suit. While he had a rich undergraduate experience, enjoying his exposure to other academic fields and meeting his wife Anne, he has called his research project then a “complete disaster.” In graduate school, Nurse had enough scientific disappointments that he sometimes contemplated leaving his lab to pursue philosophy. Fortunately, with boosts from enthusiastic teachers and supportive colleagues, Nurse stuck with it because, as he puts it, he is “very much an experimentalist.” But Nurse thinks it took him some time to realize his talent because, as he recently told the Lasker Foundation,
“when you’re an undergrad, you’re taught the most perfect experiments. When you actually do it yourself, it just fails all the time.”
Nurse credits his string of setbacks, which almost rival his long string of accolades, for readying him for the ups and downs of research. In fact, he advises early-career scientists to “fail early on because you get tougher that way.”
To this day, as the director of the Francis Crick Institute, Nurse is still trying to piece together how the collection of so-called cyclin-dependent kinases, of which cdc2 is central, carry out their business overseeing the cell cycle. But he is also working on some “slightly crazier problems” in yeast, such as what determines the size of compartments within the cell called organelles that serve specific functions, such as making proteins. “I have no idea whether they’re going to work on not,” says Nurse.
“Eureka-Style” Moments
Nurse points to two “extraordinary, eureka-style moments” in his career, which broke through his “long periods of drudgery” of experiments not working. The first was his observation of the wee yeast cells under the microscope. At the time, Nurse was searching instead for enlarged yeast cells, which would presumably be delayed in their progression through the cell cycle. He was drawn to the cell cycle and specifically how the cell cycle is controlled because, to Nurse, it seemed like the most straightforward way to study how living things reproduce and cells differentiate. Cell differentiation was one of the grand questions of the day, and ever since Nurse became convinced in undergraduate that he wanted to do biological research, he charged head-on into them. If science was so difficult, he reasoned, a ‘go big’ approach would at least yield big payoff. But, as Nurse recalls, few people at the time cared about cell cycle control, which kept his research happily free of competition.
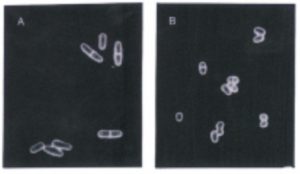
Normally-sized fission yeast cells (left), wee mutants (right)
As soon as Nurse spotted the wee cells, he started running around his post-doc lab and shouting to everyone about what he had seen. His colleagues were less impressed, suggesting the small cells could just be contamination from another organism on the Petri dish or some other kind of artifact. Their attitude, while perhaps a buzzkill, arose from the skepticism that he thinks is actually at the heart of being a good scientist. “They get evidence about a phenomenon, they get a hypothesis about what can be used to explain it, then they test it, and they test it to destruction, and if it survives then it keeps going,” he says. Before long, Nurse was able to convince himself and his advisor, Murdoch Mitchison, that the wee cells were in fact yeast, and gradually tell the story of what was happening with them.
The other eureka moment came when Nurse and Melanie Lee, a post-doc in his lab at ICRF, identified human CDC2. Against their colleagues’ and their own better judgment, they delivered a full catalog of human genes into yeast that had a defective cdc2 gene and, lo and behold, found one that could stand in for its faulty yeast counterpart.
“That was an experiment which frankly had no right to work,”
says Nurse. Yeast and humans probably split apart in evolution about 1.5 billion years ago, and although Nurse was hopeful, the idea that yeast could reveal much more than just how the cell cycle worked in yeast “seemed a bit far-fetched.” But since that fateful experiment, there have been countless experiments reaffirming the similarities between single-celled yeast, humans, and many other eukaryotes (organisms that house their DNA in a nucleus).

Nurse’s advice to students: “Fail, fail early on, because you get tougher that way. I had failed so much early on, I didn’t really take much notice of it. When you do experiments like this, which I think are a bit crazy, you know like looking for a human homolog, you do fail a lot. You have to live with those difficulties.”
“There are basic processes that are important for medicine which are conserved in all eukaryotic cells, from yeast to humans,” says Nurse, explaining why experiments in basic yeast can be important for human medicine. “Because yeast is so powerful as an experimental system, you can do everything very fast, and you can do it to a very high standard; then there is a case to be made for using yeast if you are the explorer at the front somewhere trying to find out a new [biological process]…You discover something and then others will follow and show that it’s relevant, or not relevant, to humans,” Nurse adds.
Science and Society Intersect
Despite Nurse’s exhilarating discoveries and their exhilarating implications for human medicine and understanding life, he recently admitted that “I can’t even bear to read my own scientific papers; there’s nothing inspirational about them at all.” When he first reported the wee mutant, for example, he couldn’t tell the serendipitous story, and instead, because of the style of such academic articles, had to describe the discovery like they were methodically looking for it the whole time. As much as this approach makes science boring for scientists, it can also alienate scientists from the rest of society.
“The experts have to go out there and engage. Many scientists are not very good at it, [but] we’ve got to encourage those who are good at talking to the public,”
Nurse says. Nurse chaired the Science in Society Initiative at Royal Society, which he went on to preside over from 2010 to 2015, in the hope of improving the conversation between scientists and the public.

Nurse, his postdoctoral advisor Murdoch Mitchison, and Sergio Moreno dine together in Salamanca, Spain in 2004. Mitchison was one of the first scientists to use fission yeast as a model organism. Image source: Sergio Moreno
Throughout his career, Nurse has been outspoken about the many important ways that science and society intersect, such as over scientific funding and stem-cell research, which earned him the designation of ‘most important British scientist’ in 2010. More recently, Nurse has also been sharing his own personal genetics. The story started several years after he became president of Rockefeller University in New York, a position he held from 2003 to 2010, and decided to apply for a green card. A closer inspection of his birth certificate started him on a quest that ended with him learning that the people he thought had been his parents were actually his grandparents. His mother, who he thought was his sister, got pregnant as a teenager, and to protect her reputation, the family hid the pregnancy from everyone, including Paul and his two uncles (who he thought were his brothers). By the time he figured it out, his grandparents and mother were all deceased. Nurse still does not know who his father is but says he may one day use his genetics skills to “have a go of it.”
After the initial confusion subsided, Nurse was able to appreciate the “irony” of the situation, as he told an audience at the World Science Festival in New York in 2014: “I’m not a bad geneticist, and my rather simple family kept my own genetic secret for over half a century.”
By Carina Storrs
