Even after nearly 40 years, Charles Sawyers can't forget the patient he treated during his medical residency at the University of California, San Francisco (UCSF). The patient, a firefighter in his 20s, had the blood cancer chronic myeloid leukemia (CML), which usually affects people decades older. "He was younger than I was," says Sawyers, now an oncologist at Memorial Sloan Kettering Cancer Center (MSKCC).
In the 1980s, CML patients typically survived only 4 or 5 years. However, the firefighter was young enough to receive a bone marrow transplant. That treatment, which only about 10% of patients qualified for, could cure his cancer. Instead, the side effects killed him. His death came as a shock not just because of his age but also because leukemia patients spent long periods in the hospital and "you really got to know them," Sawyers says. "There's a connection that is quite intense." The patient had received what was then the state-of-the art treatment but had died anyway. "There's got to be a better way," Sawyers thought at the time.
Sawyers presents his remarks at the 2009 Lasker Ceremony.
Sawyers shared the 2009 Lasker~DeBakey Clinical Medical Research Award for finding that better way. He was part of the team that launched the drug imatinib (Gleevec), which transformed CML therapy. "All of the sudden we could give a pill to almost everyone [with the disease]," says oncologist Jeff Lipton of the University of Toronto, who wasn't involved in the drug's initial development.
"The impact was huge."
Still, some patients relapsed because their cancer cells became resistant to the drug. Sawyers figured out why, and his research led to another new drug, dasatinib (Sprycel), for treating such patients. He then repeated the feat for prostate cancer, discovering a drug-resistance mechanism that spawned two new treatments for that disease. "Charles has a brilliant scientific mind," says oncologist Brian Druker of the Oregon Health and Science University, who shared the 2009 Lasker Award with Sawyers. "He has this capability of looking at a problem carefully and thoughtfully and identifying what the right point for interventions will be."
Digitized colored light micrograph of cancerous blood cells in chronic myeloid leukemia
Courtesy of Cecil Fox / Science Source
Joining the Family Business
Sawyers was born in 1959 in Nashville, Tennessee, and grew up there. His parents were doctors, as were three uncles and one grandfather. With that family background, "it was assumed I would be a physician," he says. As an undergraduate at Princeton University, however, he chose to major in history. He wasn't rejecting a medical career, but he wanted to explore other options and "make sure I wasn't closing any doors." Sawyers enjoyed the liberal arts classes he took and would have been happy as a historian, he says, but his older cousin, also a doctor, persuaded him to apply to medical school at Johns Hopkins University.
At first, Sawyers thought he'd made a mistake. The first-year curriculum was all rote learning—students had to memorize large numbers of facts and then regurgitate them on exams. "I knew I could survive, but it wasn't enjoyable," Sawyers says. During the summer break, he took a newspaper internship and considered dropping out of medical school to become a science writer.

Sawyers (far right) and his UCLA lab members in 1995, when they were investigating BCR-ABL.
Courtesy of Charles Sawyers
He stayed in medical school, however, and his second year was more interesting and satisfying because "I got bitten by the science bug," he says. As he discovered, some doctors also were researchers, a combination he found enthralling. Sawyers, a self-described late bloomer, had finally discovered what he wanted to do—and had a knack for it. "I realized I had this problem-solving curiosity that scientists need to have."
After graduating in 1985, Sawyers started his residency at UCSF, where he worked in the hospital wards at night and performed lab research during the day. The CML patients he treated during his rotations sparked his interest in the disease. Thanks to the findings of Peter Nowell of the University of Pennsylvania School of Medicine and Janet Rowley of the University of Chicago, winners of the 1998 Albert Lasker Clinical Medical Research Award, researchers knew that the trigger for CML was a cellular blunder that causes chromosomes 9 and 22 to swap their tips. That exchange creates a combo gene that codes for a rogue protein, BCR-ABL, that drives the patient's bone marrow to churn out excess white blood cells.
When Sawyers finished his residency, he wanted to investigate CML. But he needed to improve his research skills. "If you know you have the drive and the passion, you still need a mixture of practical and intellectual tools" to do good science, he says. To gain lab experience, Sawyers took a position as a postdoctoral researcher at the University of California, Los Angeles (UCLA). He continued to study the disease after the university hired him as a faculty member in 1993.
Countering Cancer's Countermeasures
Sawyers's career took a turn a couple of years later when his friend Druker asked for help. Druker and the other 2009 Lasker Award recipient, Nicholas Lydon, then at the Swiss drug company Ciba-Geigy, had come up with a drug that thwarted BCR-ABL. The first clinical trial of the drug, imatinib, was about to start, and Druker and colleagues wanted Sawyers to participate. "I could not think of anyone better than Charles," Druker says. "He knew CML inside and out."
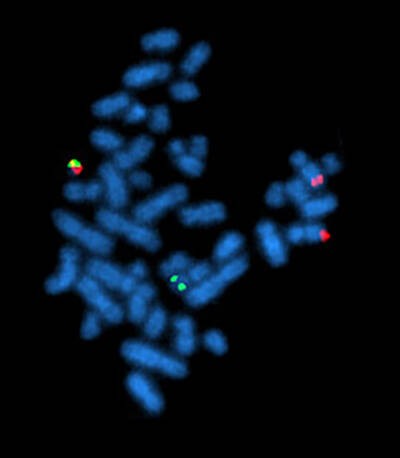
Fluorescence in situ hybridization, showing the rearrangement of BCR and ABL genes in the chromosomes of a cell. Philadelphia chromosome can be seen top left with both green and red (BCR-ABL fusion gene) fluorescence.
Courtesy of Wikimedia Commons
The trial's results, described in two 2001 papers in the New England Journal of Medicine, showed that imatinib had a powerful effect on the disease. In 53 of 54 patients who received the highest doses, the number of white blood cells fell to normal levels. The drug worked even in patients near death. Imatinib, which the Food and Drug Administration (FDA) approved in 2001, also was easier for patients to tolerate than treatments such as bone marrow transplants. For some patients in the study, the drug yielded long-term benefits. Sawyers says that he regularly chats with a participant who started taking the drug in 1999.
Still, even before the trial was over, researchers had noticed that CML often rebounded as patients' cells became resistant to the drug. "Charles's biggest contribution was understanding the resistance to imatinib," Druker says. To find out why cells stopped responding to the treatment, Sawyers and colleagues sequenced the gene for BCR-ABL in patients who had developed resistance. The researchers pinpointed 15 changes to the protein. Initially, that result seemed like bad news. To fight resistance, scientists might have to develop multiple drugs to target the diversity of mutations. But the picture changed when Sawyers's lab teamed with other researchers to determine how the mutations changed BCR-ABL's structure. Many of the mutations, the scientists discovered, triggered a similar effect, reshaping the protein so that the drug couldn't fit in. That result was good news because it implied that a drug that could bind to the distorted BCR-ABL would combat many resistance-inducing mutations. All the scientists had to do was find such a drug.
By chance, that molecule already was sitting on the shelves at the drug company Bristol-Myers Squibb. In the early 2000s, a scientist there phoned Sawyers to tell him that the company had developed several compounds that might stymie BCR-ABL. In lab studies, Sawyers and colleagues found that one of those molecules, dasatinib, inhibited BCR-ABL proteins with most alterations that caused resistance to imatinib. Clinical trials of dasatinib showed its value for patients with CML, leading to FDA approval of the drug in 2006. "It's hard to overstate the importance" of what Sawyers, Druker, and colleagues achieved, says oncologist Ross Levine, also of MSKCC. Patients are taking imatinib and dasatinib today, along with newer drugs that also target BCR-ABL. Those treatments allow most patients with CML to live about as long as people without the cancer.
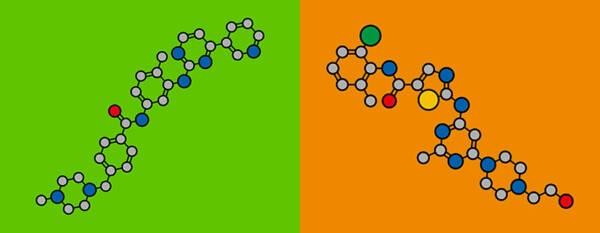
Stylized chemical structure of imatinib (left) and dasatinib (right); atoms are shown as color-coded circles: carbon (grey), oxygen (red), nitrogen (blue), sulfur (yellow), chlorine (green).
Courtesy of MOLEKUUL / Science Source
Beyond CML
When Sawyers began his research on mutations in CML, some scientists suspected that imatinib resistance stemmed from mechanisms that didn't involve changes in BCR-ABL. For example, cells might switch on pumps that eject the drug. Sawyers's findings were significant, showing that mutations that altered the target of the drug led to resistance, Levine says. "That is obvious now, but at the time it was extraordinary." That insight allowed researchers to discover a range of new drugs to overcome resistance in other cancers.
Sawyers led the way. After studying CML for more than 10 years, he switched to prostate cancer. "I wanted to take on a new challenge," he says. Moreover, almost 300,000 new cases of prostate cancer occur in the United States every year, versus only about 9,000 for CML, so any discoveries he made could have a larger influence on patients' health. The androgen receptor—the molecule that allows cells to respond to testosterone and other hormones—is one culprit in prostate cancer because the receptor spurs tumor growth. Patients often receive drugs that reduce their testosterone levels or block the receptor. But prostate tumors can become resistant to those treatments.
In 2004, Sawyers and colleagues figured out why. Cells from resistant tumors use a simple trick—they crank out more of the androgen receptor. Sawyers says he tried to persuade pharmaceutical companies to pursue drugs to defeat that countermeasure, but they weren't interested. Instead, his lab joined forces with a UCLA chemist, Michael Jung, and colleagues to discover their own compounds.
The researchers reasoned that the best strategy for thwarting resistance was a stickier molecule that would adhere tightly to excess androgen receptors. As the researchers revealed in 2009, they pinpointed two candidates that bound to the receptor up to eight times more tightly than a standard prostate cancer medication. One of those molecules became the drug enzalutamide (Xtandi), which the FDA okayed in 2012. Sawyers's research yielded another new drug, apalutamide (Erleada), that also targets the androgen receptor and was approved in 2018.

Sawyers (left) with his co-winners, Brian Druker (middle) and Nicholas Lydon (right) holding their statuette at the 2009 Lasker Awards Ceremony
Along with his own investigations, Sawyers has boosted cancer research in other ways. In 2006, he moved from UCLA to MSKCC to head a new effort, the Human Oncology and Pathogenesis Program, for speeding development of cancer treatments. "He made an impact and paved the way for others to make an impact," Levine says.
Sawyers's fellow researchers attribute his success to several qualities. "Dogged" is one adjective that describes him, Levine says. "He brings extraordinary focus to the question he is studying." Druker lauds Sawyers's ability to solve problems, which stems from his scientific acumen. "It's knowing the right questions to ask and making sure you are asking these questions," he says.
With all that he has achieved, Sawyers could retire and spend more time running marathons, camping, and hiking in the Sierra Nevada mountains. But he says he plans to continue his research because prostate tumors have many ploys to elude treatments. For example, the cells can change their identity so that they don't require the androgen receptor, Sawyers says. "It's kind of devious." He and colleagues are trying to determine how cells pull off the switch. "The last act of my career will be to come up with ways to prevent that escape route," he says.

In 2019, Sawyers (second from right) and his wife, Susan (right), met with one of the first patients to take Gleevec, Virginia Garner (shown here with her husband, Van). Garner received her first dose of the drug 20 years prior.
Courtesy of Charles Sawyers
Sawyers's findings have helped CML and prostate cancer patients survive longer. But by uncovering how resistance occurs and how to combat it, he made a broader impact on research into CML and on patients' lives. "He laid the foundations for what we do for virtually every other cancer," Lipton says.
By Mitchell Leslie


