Unanticipated revelations, new biological horizons
As a graduate student in the early 1960s, Baltimore wondered whether mammalian viruses might help scientists understand human physiology the way bacterial viruses had helped them understand E. coli. By following this instinct, he has unearthed a treasure chest of knowledge.
Two years after joining the Massachusetts Institute of Technology in 1968 as a junior faculty member, Baltimore discovered that certain tumor viruses can copy their genetic material, RNA, into DNA. This capability violated conventional wisdom, which held that genetic information flows from DNA to RNA to protein. He tracked this dogma-defying feat to an enzyme, now called reverse transcriptase, and published the work in 1970 back-to-back with the late Howard Temin (University of Wisconsin, Madison), who had posited the idea and independently performed similar work. For this achievement, they won the 1975 Nobel Prize for Physiology or Medicine (Figure 1). Baltimore was 37 years old.

Receiving the Nobel Prize from Swedish King Carl Gustaf in 1975
This advance delivered numerous practical and theoretical impacts, some of which were not yet known at the time. Eventually, for example, reverse transcriptase enabled the isolation of protein-coding portions of genes, a task that was difficult to accomplish from genomic DNA, which contains long sequences that interrupt the protein template. The enzyme allowed scientists to make a DNA copy of mature messenger RNA, in which these stretches had been spliced out by the cell’s machinery.
Baltimore wanted to use viruses to study cancer in mammals. Toward that end, he began studying the Abelson virus, which causes leukemia in mice and corrupts well-behaving mouse cells in culture dishes, goading them to proliferate wildly. Baltimore and postdoctoral fellow Owen Witte traced its cancer-causing abilities to a previously unknown enzymatic power—the capacity to add phosphate chemical groups to the amino acid tyrosine within proteins. Tony Hunter (Salk Institute of Biological Studies, La Jolla) independently made similar observations in other viruses. Such chemical adornments control signaling systems that regulate a variety of activities, including cellular replication.
This finding led to a cascade of discoveries, some of which have culminated in medical benefits. Most notably, a defective version of a related human enzyme underlies chronic myelogenous leukemia, and scientists developed a compound that blocks its activity. The resulting drug, imatinib (now widely known as Gleevec), has transformed this previously fatal illness into a manageable condition and saved many lives (Lasker Clinical Research Award, 2009).
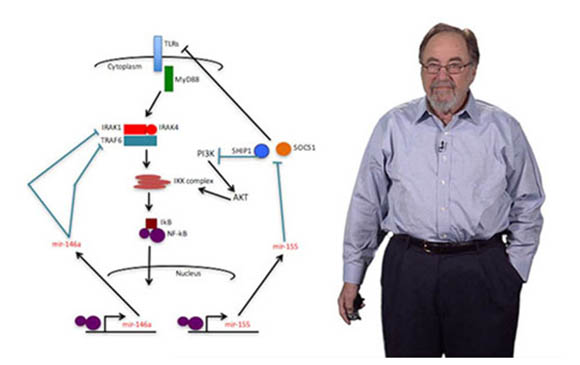
Baltimore then harnessed numerous other observations about Abelson virus’s effects on cells in culture to expand his studies into developmental immunology. He uncovered a protein, NF-kB, that activates hundreds of genes involved in inflammation and plays key roles in many diseases, particularly numerous cancers.
Later, Baltimore found genes whose products orchestrate one of the most awe-inspiring developmental processes in existence. All B cell precursors contain the same set of genes, yet somehow each mature B cell acquires the ability to make a unique antibody that recognizes a specific foreign peptide. With Baltimore, graduate students David Schatz and Marjorie Oettinger devised and deployed a scheme by which they isolated the two so-called Recombination-Activating Genes—RAG-1 and RAG-2—that rearrange antibody genes to generate an astounding degree of diversity from a constant starting set of genetic information. Baltimore also proposed how an enzyme called terminal transferase might help generate diversity in antibody genes, and this theoretical work opened untilled ground.
Each of these breakthroughs has spawned a new field, yet they represent only a subset of Baltimore’s major findings. He has published more than 700 papers, many of which hold a central place in their discipline.
Training students and postdoctoral fellows has been integral to Baltimore’s research accomplishments. He believes that the best young scientists will flourish if they receive financial and intellectual support as well as the latitude to follow their own ideas. With this philosophy, he has mentored scores of scientists, many of whom are now world renowned. Twenty of his scientific progeny have become National Academy of Sciences members.
Deft institutional leadership
In 1980, a wealthy entrepreneur named Jack Whitehead invited Baltimore to help him build a new biomedical research institute. Baltimore crystallized Whitehead’s general notion into a clear vision, and then made it a reality, thus creating one of the best centers for experimental biomedicine on the planet. Along the way, he convinced Whitehead that MIT would be its ideal home even though the school was known more for mathematical and technical prowess than for health sciences. Then, against much suspicion and skepticism, he convinced MIT to accept it into the heart of the school. He enticed top-flight investigators who were probing exciting questions and melded these personalities into a functioning institution.
In 1982, he became the founding director of the Whitehead Institute for Biomedical Research (Figure 2). Its relationship with MIT became a model for other institutes, and he also conceived a novel—and now emulated—program that offers especially talented recent PhD and MD-PhD graduates the opportunity to pursue independent projects without first apprenticing as a postdoc.

With Jack Whitehead during construction of the Whitehead Institute in 1984
In addition to launching this new institution, Baltimore has led proven and esteemed academic establishments. Even his brief stint as President of the Rockefeller University (1990-1991) left a long-lasting and constructive imprint. He raised the status of junior faculty, unlocking them from a hierarchical model in which they struggled to thrive. In the same position at California Institute of Technology (1997-2006), he raised the largest ever single donation at the time to support science at a US university. This gift provided seed money for imaginative, interdisciplinary initiatives and gave all of the divisions at Caltech the opportunity to imagine new directions for themselves.
For the public good
When the US invaded Cambodia in 1970, Baltimore was pipetting away, performing his reverse transcriptase experiments. Compelled to stand against an immoral and illegal war, he froze his samples and joined the protests. Since then, Baltimore has comingled his intellectual acumen, personality, and moral compass to contribute, away from the bench, to the scientific community and society (Figure 3).

Accepting the National Medal of Science from President Bill Clinton in 2000
The recombinant DNA revolution began in the early 1970s and concern immediately emerged about possible hazards. Baltimore felt called to figure out how to respect the ethical issues without halting research. He worked with Paul Berg, Maxine Singer, and others to organize the famous 1975 Asilomar Conference, whose participants produced a strategy for engaging prudently with the new technology while exploiting it to engender knowledge. Baltimore subsequently served on the Recombinant-DNA Advisory Committee of the National Institutes of Health (NIH).
Four decades later, gene-editing tools became available, and his peers again turned to him for leadership as they began to cultivate a discussion about how to navigate this unexplored territory. He chaired the first (2015) and second (2018) International Summits on Human Gene Editing, which grappled with questions that are arising from the impending possibility of manipulating human genes in a heritable manner—a venture that presents enormous benefits, such as correcting genetic disorders, as well as risks.
Baltimore has also helped galvanize the US to tackle a serious medical problem that the federal government was ignoring. In 1986, the HIV/AIDS epidemic was raging, yet no national program existed through which to plan a response. The National Academy of Sciences and the Institute of Medicine asked Baltimore and the late Sheldon Wolff (Tufts University School of Medicine) to co-chair a commission that would study the relevant issues. The resulting report reshaped the country’s approach to the scourge. It injected a sense of urgency into the situation and recommended a monumental research, educational, and public health campaign to learn about the virus and curb the spread of disease. Baltimore had the gumption to call for a $1 billion research program—a tremendous amount of money at the time.
Baltimore shines as a model for all facets of the scientific enterprise. He has made breakthroughs in manifold fields, nurtured rising investigators, grown institutions and programs from scratch, and led with wisdom. His thoughtfulness and integrity have guided colleagues and the public, and his ever-expanding legacy continues to benefit the world of biomedicine and society at large.
by Evelyn Strauss
Selected Publications of David Baltimore
Baltimore, D. (1970). Viral RNA-dependent DNA polymerase. Nature. 226, 1209-1211.
Baltimore, D. (1974). Is terminal deoxynucleotidyl transferase a somatic mutagen in lymphocytes? Nature. 248, 409-411.
Witte, O.N., Dasgupta, A., and Baltimore, D. (1980). Abelson murine leukemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 283, 826-831.
Sen, R., and Baltimore, D. (1986). Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 46, 705-716.
Baltimore, D., (2009). Discovering NF-kB. Cold Spring Harb. Perspect. Biol. https://cshperspectives.cshlp.org/content/1/1/a000026
Zhang, Q., Lenardo, M.J, and Baltimore, D. (2017). 30 Years of NF-kB: a Blossoming of relevance to human pathobiology. Cell. 168, 37-57.
Schatz, D.G., Oettinger, M.A., and Baltimore, D. (1989). The V(D)J recombination activating gene, RAG-1. Cell. 59, 1035-1048.
Schatz, D.G., and Baltimore, D. (2004). Uncovering the V(D)J recombinase. Cell. S116, S103-S106.
Baltimore, D. (2019). Sixty years of discovery. Annu. Rev. Immunol. 37, 1-17.
Perspective
Crotty, S. (2001). Ahead of the Curve: David Baltimore’s Life in Science. University of California Press, Berkeley.