Joel Habener
Massachusetts General Hospital

Lotte Bjerre Knudsen
Novo Nordisk

Svetlana Mojsov
The Rockefeller University
For the discovery and development of GLP-1-based drugs that have revolutionized the treatment of obesity
The 2024 Lasker~DeBakey Clinical Medical Research Award honors three scientists for their discovery and development of GLP-1-based drugs that have revolutionized the treatment of obesity. Joel Habener (Massachusetts General Hospital) and Svetlana Mojsov (The Rockefeller University) discerned the physiologically active form of the hormone, and Lotte Bjerre Knudsen (Novo Nordisk) turned it into medications that promote weight loss.
Globally, almost 900 million adults are living with obesity. In the United States, it afflicts as many as 40% of adults; in Europe the prevalence approaches 25%. The excess pounds underlie multiple life-threatening conditions. Obesity is commonly viewed as a failure of willpower, yet for many, diet and exercise don’t cure the problem. Historically, attempts to make safe and effective drugs that help people slim down have fallen short. Habener, Mojsov, and Knudsen have introduced a new era of weight management in which GLP-1-based pharmaceuticals promise to dramatically enhance health.
The boogie-woogie approach to creativity in art and science
The Dutch painter Piet Mondrian, famous for his geometric grid paintings, was an ardent jazz fan. In the late stage of his career, he became intrigued with boogie-woogie music, which inspired him to paint his masterpiece, Broadway Boogie Woogie.
Award Presentation: Richard Lifton
Obesity is a major public health problem affecting more than 40% of U.S. adults and 1 billion people worldwide. This is literally a growing problem; since 1960, average adult weight in the US has increased more than 30 pounds, attributed in part to reduced physical activity and ready availability of highly processed foods that are dense in calories.
Obesity has a significant impact on health and longevity. It is the major risk factor for type 2 diabetes, defined by chronic elevation in blood glucose levels; 90% of the 38 million type 2 diabetics in the US are overweight.
These considerations set the stage for today’s Lasker~Debakey Clinical Medical Research Award, as we recognize three scientists, Svetlana Mojsov, Joel Habener and Lotte Knudsen for their remarkable discoveries that have resulted in the development of revolutionary medicines that safely lower blood glucose in diabetics and induce profound weight loss that reduces obesity’s comorbidities.
The story starts 1906 with a report that an extract of intestinal tissue could lower blood glucose, but no progress was made toward purifying this factor. The story was revived in the 1960s when oral administration of glucose was found to produce a greater increase in insulin secretion than the same dose administered intravenously, suggesting that release of a gut hormone was priming the pancreas for insulin secretion in response to a meal. But the responsible factor, a so-called incretin, remained elusive.
The development of recombinant DNA technology in the 1970s provided new opportunities for gene discovery and connected the paths of two of today’s honorees. Svetlana Mojsov, as a graduate student with Bruce Merrifield at Rockefeller University, developed novel methods of solid phase peptide synthesis that enabled her to synthesize the short peptide hormone glucagon, which protects against hypoglycemia. In parallel, Joel Habener, an endocrinologist at MGH, cloned DNA copies of the messenger RNA encoding glucagon from the anglerfish. He found that the glucagon peptide was embedded in a longer precursor protein and snipped out by specific cuts. Surprisingly, he found that the precursor protein included another peptide sequence related to the glucagon family that was previously unidentified.
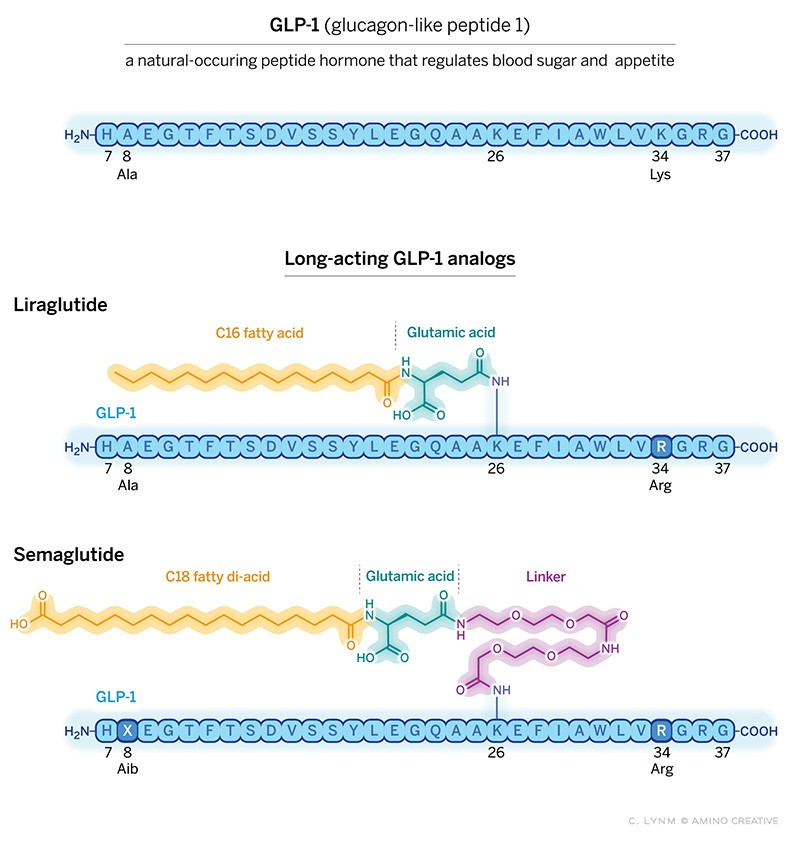
The following year, the homologous gene from hamster was cloned, and glucagon was again embedded in a larger precursor protein along with another potential peptide that was very similar to the anglerfish peptide; this peptide was named GLP-1.
As fate would have it, the hamster gene publication coincided with Svetlana Mojsov’s arrival on the faculty of MGH as director of the peptide synthesis facility. Svetlana dove into these publications with several critical questions- what was the active form of GLP-1, where was it produced, and what does it do? Svetlana’s experience led her to deduce that the active peptide would comprise 31 amino acids. She then used her skills to synthesize large quantities of the 31 amino acid peptide and made antibodies to it. She and Habener teamed up to show that the peptide was produced in the intestine; they published these results in 1986. It did not escape their attention that this peptide might be the long-sought incretin hormone.
To test this idea, Mojsov and Habener collaborated with Gordon Weir, showing that miniscule quantities of this peptide dramatically increased insulin secretion by the rat pancreas. Related findings were published by Holst in Copenhagen. Habener’s postdoc Daniel Drucker, Mojsov and Habener also showed that GLP-1 induced glucose-dependent insulin secretion from a pancreatic islet cell line. A story was emerging. Infusion of GLP-1 into healthy humans and diabetics by several groups established the role of GLP-1 as an incretin and a potential therapeutic for diabetes.
There was, however, a challenge: the half-life of GLP-1 in plasma was only 1-2 minutes. A much longer half-life would be essential. Enter Lotte Knudsen, a medicinal chemist at Novo Nordisk, determined to not just to develop a drug for diabetes, but to also determine whether GLP-1 might be another long-sought drug—one that safely and effectively causes weight loss. This speculation derived from the discovery by Stephen Bloom in London that injection of GLP-1 into the intracerebral ventricles strongly inhibited feeding in fasted rats. Thus began a saga that saw Knudsen’s teamwork for nearly 20 years to maximize the half-life of GLP-1.
In 2010 Novo Nordisk gained FDA approval for once-a-day subcutaneous injection of a modified form of GLP-1, liraglutide. Liraglutide also produced ~5 lb. weight loss, giving Knudsen and her team the encouragement to press on. Over the next 5 years, after testing thousands of modifications, the team reported the development of semaglutide, a highly modified GLP-1. Semaglutide was strong enough binding to albumin in plasma to prevent its degradation and filtration by the kidney while preserving robust activation of its pancreatic receptors to stimulate insulin secretion. This chemical tour de force increased semaglutides half-life two thousand fold, allowing once-weekly subcutaneous injection. In 2017, semaglutide received FDA approval for treatment of diabetes at a dose of 1 mg. per week.
But the biggest surprise came when the dose was increased from 1 mg to 2.4 mg per week: not only was blood glucose control excellent, but weight loss was astounding – obese people without diabetes lost on average 15% of their starting weight (more than 30 pounds in a person starting at 220 pounds). This led to FDA approval of high dose semaglutide for weight loss in 2021.
This weight reduction was not merely cosmetic. Placebo- controlled trials of semaglutide in people with existing cardiovascular disease reduced CV death, heart attack or stroke by 20%. Similarly, In diabetics with progressive kidney failure, treatment significantly reduced progression and the need for dialysis or kidney transplantation. Promising results have also been seen in obesity-related fatty liver with fibrosis. Recently, a real-world observational study comparing GLP-1s to insulin for diabetes showed that GLP-1s reduced the incidence of 10 different obesity-related cancers by 25% to 65%. GLP-1s are also being tested in diseases ranging from Parkinson’s disease to a range of compulsive or addictive behaviors. Better understanding of GLP-1’s mechanism of action and how to best sustain weight loss are matters of intense investigation.
Word of these dramatic results spread like wildfire, and uptake of GLP-1s has been meteoric. Semaglutide has been taken by literally millions of people in the US in the last three years.
To no one’s surprise, the rest of the biotech and pharmaceutical industry has noticed. Eli Lilly has developed tirzepatide, which adds activity of another incretin-like peptide, GIP, to the GLP-1 backbone. Tirzepatide produced a 21% average weight reduction in non-diabetics and was FDA approved for weight loss in 2023. Oral versions of GLP-1s have also been approved, though they thus far have lower efficacy.
It is hard to overestimate the potential of these remarkable discoveries for human health. In their 1968 recording of Revolution #1 the Beatles’ John Lennon sang, “You say you want a revolution, well you know, we all want to change the world.” GLP-1s and their successors are well on the road to changing the world, and Svetlana Mojsov, Joel Habener and Lotte Knudsen are recognized today as the revolutionaries who lit the match. Congratulations to this year’s recipients of the Lasker~DeBakey Clinical Medical Research Award.
Acceptance remarks
Acceptance Remarks: Joel Habener
As I look back on my nearly 60 years of biomedical research, I realize how important serendipity was in shaping of my career in research.
My first unanticipated serendipitous event occurred in 1967 when, because of the outbreak of the Vietnam war, I was drafted into the US Public Health Service and assigned to the NIH to undertake two years of laboratory research on the mechanisms of DNA replication. While I was at the NIH, the first studies were just beginning that inaugurated the field of molecular biology. My experience at the NIH was a tremendous inspiration.
The next serendipitous event occurred while I was at the NIH when I met John Potts, who invited me to join his staff in his newly-appointed position as the chief of the endocrine unit at the MGH. I obtained a Special NIH Fellowship to study the processes of the biosynthesis of parathyroid hormone. However, a problem arose–the Endocrine Unit was not yet equipped to conduct the studies.
Again, purely by chance, serendipity intervened. I was introduced by a mutual friend to Alexander Rich, a molecular biologist at MIT, who invited me to join his lab and to work collaboratively with John Potts and a post-doc, Byron Kemper. Our studies led to my appointment as an investigator in the Howard Hughes Medical Institute, which supported my work at the MGH for 30 years.
With the support of the HHMI, I decided to investigate the causations of type 2 diabetes, a disorder of insulin resistance. My newly-recruited post-docs Kay Lund and Dick Goodman and I planned to use recombinant DNA technology to clone the gene for glucagon using the hormone-producing islets isolated from the rat pancreas. But our plans were upset by a moratorium on recombinant DNA studies in warm-blooded animals. Again, serendipity intervened. We learned that fish, cold-blooded animals, make their pancreatic hormones in an organ that is separate from the exocrine pancreas (Brockmann bodies), thus greatly facilitating the preparation of high-quality messenger RNA to prepare the cDNA for cloning. The use of the Anglerfish Brockmann bodies greatly facilitated our work that identified the fish homolog of GLP-1.
Acceptance Remarks: Lotte Bjerre Knudsen
I am truly honored to receive this award, and I want to offer my profound gratitude to the Lasker Foundation and the jury for including me. The Lasker Foundation celebrates scientific and medical advances, and that is very important. The story of GLP-1 fits perfectly within this.
Science is rarely a solitary endeavor. Great scientific breakthroughs demand perseverance, a willingness to embrace risk, and to work effectively and selflessly with others. In accepting this award, I do so on behalf of countless brilliant individuals who have been part of this journey.
Most of my scientific journey has unfolded over 35 incredible years at Novo Nordisk. I was responsible for the invention of the first long-acting GLP-1, and proposed we should focus on obesity as well as diabetes. At the time, the notion that one molecule could have two separate effects was provocative, as was the thought of having a novel injectable medicine for these conditions. I passionately believed in the molecule and its potential for both diseases, and I have continued to be a part of the unfolding scientific narrative surrounding GLP-1 over those 35 years.
This award gives me a chance to highlight the work we do in the pharmaceutical industry. The case of GLP-1 illustrates how numerous inventions were needed before we arrived at a medicine that induces double-digit per cent weight loss as well as reduced cardiovascular disease risk in obesity. Aside from the inventions, countless people did and still do important work to generate the clinical evidence required, and to get these medicines to the people who need them. Especially when it comes to developing medicines for obesity; I have spent more than three decades in a company that encouraged scientific discoveries in several diseases, including diabetes and obesity, and progressed them at a time when few companies believed in medical treatment for obesity, or indeed in GLP-1.
Outside the pharmaceutical industry, many leaders in medicine continue to advocate for obesity as a serious chronic disease, and for access to evidence-based treatments to reduce the burden patients face, much work still needs to be done, and these medicines may serve as a catalyst in the dialogue.
Breakthrough discoveries require vision as well as courage. By their very nature, they only materialize at the end of a long process—when odds, obstacles and objections have been overcome. My message to all aspiring young scientists is to believe in your ideas and pursue them with determination. Never be afraid to suggest something novel. A life where scientific thinking guides you is always a truly exciting one.
Acceptance Remarks: Svetlana Mojsov
I am very honored to receive the Lasker~DeBakey Clinical Medical Research Award for my contribution to the discovery of GLP-1.
Forty-one years ago, on November 4, 1983, I began to synthesize GLP-1 peptides by the solid phase method in my Laboratory at the Endocrine Unit in the Bulfinch Building in Massachusetts General Hospital in Boston. All my syntheses were done on a manual shaker invented by my PhD mentor Bruce Merrifield in the 1960s, when he developed the solid phase method for the synthesis of peptides and proteins that forever changed the field of peptide chemistry. In 1983, manual shakers were not available commercially. They were made by the Instrument Shop at the Rockefeller University and Dr. Merrifield sent me one from his laboratory.
That was the beginning of a scientific journey that involved collaborations between large number of chemists with biologists and clinicians—first in academia, and later in pharmaceutical companies. Our joint efforts led to the development of GLP-1 analogs for diabetes and obesity.
It is my privilege to have been there at the very beginning.
Thank you very, very much.