H. Michael Shepard
Genentech

Dennis J. Slamon
University of California, Los Angeles

Axel Ullrich
Max Planck Institute of Biochemistry
For their invention of Herceptin, the first monoclonal antibody that blocks a cancer-causing protein, and for its development as a life-saving therapy for women with breast cancer
The 2019 Lasker~DeBakey Clinical Medical Research Award honors three scientists—H. Michael Shepard, Dennis J. Slamon, and Axel Ullrich —who invented Herceptin, the first monoclonal antibody that blocks a cancer-causing protein, and developed it into a life-saving therapy for women with breast cancer. The innovation reduces the risk of recurrence and extends survival time for patients with metastatic as well as early-stage disease. Every year, more than 50,000 women in the US are diagnosed with the type of breast cancer that the drug attacks, and over 2.3 million individuals have received the treatment since it became available. Shepard (now at BetterOutcomes4Cancer) and Ullrich (now at Max Planck Institute of Biochemistry, Martinsried, Germany) conducted their Herceptin investigations at Genentech. Slamon did his at the University of California, Los Angeles (UCLA), where he continues to work.
In the mid 1970s, scientists discovered that genes within our own bodies can trigger cancer. This revelation sparked the idea that stifling the activities of such oncogenes might thwart the trouble they instigate. This approach—in which a therapy would fixate on molecules that dwell specifically in cancer cells and also drive malignancy—held great appeal. Such a targeted strategy might avoid many of the harsh side effects associated with chemotherapy while striking the source of the affliction.
Seurat’s Dots: A Shot Heard ‘Round the Art World
How the color theories of a chemist have ricocheted across centuries of artistic and scientific imagination.
Award presentation by Michael Brown
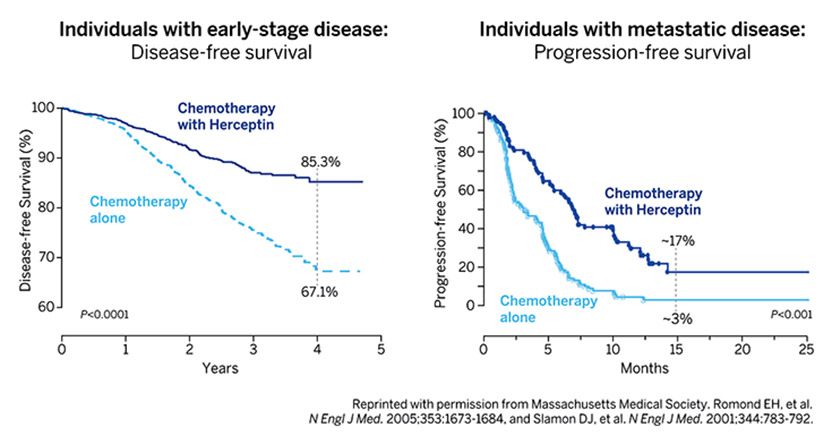
Each year 250,000 American women are diagnosed with breast cancer. Twenty percent are caused by extra copies of a gene called HER2. Until recently the 50,000 women with HER2-positive breast cancer had a terrible prognosis. Today the finding of HER2 is good news. When a breast biopsy is HER2 positive and the cancer has not advanced beyond regional lymph nodes, that woman has a 90% chance of being tumor-free for at least 3 years and likely forever. These cures come from an antibody called Herceptin that targets HER2. When Herceptin was approved in 1998 it was the first monoclonal antibody against an oncogene. This single drug changed our entire approach to cancer therapy. It emerged from the work of 3 scientists: Axel Ullrich and Michael Shepard of Genentech, and Dennis Slamon of UCLA.
A Lasker Award for cancer is appropriate because the invention of Herceptin can be traced to our patron saint Mary Lasker. Throughout the 1960’s Mary stalked the lobbies of Congress and the White House advocating fiercely for cancer research. Her lobbying bore fruit in 1971 when President Nixon launched the War on Cancer, and Congress appropriated 1.6 billion 1971 dollars.
Acceptance remarks
Acceptance remarks, 2019 Lasker Awards Ceremony
To be recognized by a Lasker award is amazing. To have this recognition together with the other awardees, like Dennis and Axel, is humbling. To have had a part in such an innovative project, which has done so much good for so many people, is a bit overwhelming. More than 2 million patients have been treated, many of whom have benefited tremendously. Herceptin is a breakthrough drug and led the way for other highly targeted therapies. Just the same, even though it has done much good and changed many lives for the better, it is not perfect. As a scientist deeply committed to this kind of work, I often remind myself, involuntarily, that although as many as 15% of breast cancer patients are helped by Herceptin, the majority are not. There remains a lot to be done, and I think that those of us who have suceeded to the extent we have done, must now be deeply involved with the next generations of scientists and clinicians who will carry this work forward. There is a lot of mentoring to do. Among other things, we must teach that having really big goals, being a dreamer, is a good thing. Young people must believe that. Perhaps right now more than in other times.
The Herceptin project evolved from an idea to create a safe cancer therapeutic. Many have come before us, and hopefully others will follow. We all had to overcome scientific difficulties…and myths. One of the most interesting myths for Herceptin was that monoclonal antibodies could not penetrate solid tumors. Luckily, the rigorous scientific environment of Genentech allowed us to directly address this issue and overcome it. We learned many other things during the course of this work, among them that the aggressiveness of HER2 tumors is in part due to creating tumor cell resistance to immune surveillance. Gail Phillips, who is here today, discovered that once tumor cells become resistant to macrophage killing, they can be subsequently stimulated by these immune cells. This is an example of how tumor cells can actually pervert antitumor responses to their benefit. Another thing that we learned during the whole process of creating Herceptin is that collaborations and communications between scientists, whether academic or biotech, drives breakthrough drug discovery and development.
Finally, it is difficult to see how I came to this place. The only explanation I can offer is that there were people along the way who believed in me, sometimes more than I believed in myself. Some of these people are here today, including George Malacinksi, my PhD mentor, and Marc Feldmann, with whom I have shared many years of scientific discussions and fellowship. I would like to thank them and all of my colleagues.
Acceptance remarks, 2019 Lasker Awards Ceremony
When Axel Ullrich, Mike Shepard and I first began the research journey that brought us here today, sharing this incredible honor, we actually shared very little else. However, what we did have in common was the intense curiosity that drives scientists to address questions of interest to them. Axel had an intense desire to identify, clone, characterize and understand the function of genes regulating cell growth. Mike was very interested in understanding how some cancers respond to innate host immunity mechanisms while others are resistant. My interests centered around two questions; first, why was there such diversity in outcomes for patients who supposedly had the same disease, i.e. lung cancer or colorectal cancer or breast cancer when treated with the “one-size-fits-all” therapies then used for malignancies arising in a given organ site; and second, what role if any did the new class of genes known as “oncogenes” play in these diseases.
Clichés frequently exist because they represent truth. That is clearly true with the saying, “those who accomplish something frequently stand on the shoulders of others.” In my case, that is a gross understatement. Each of us owe much of what we are being honored for today to what we learned from the work of others. I was heavily influenced by a mentor, Janet Rowley, who taught me that recurrent changes seen in the DNA of many malignancies are not just random noise secondary to instability of cancer genomes but can yield important information about the genesis and behavior of the diseases in which they occur. In Werner Kirsten’s lab, I worked with the Kirsten sarcoma virus, an acutely transforming retrovirus which was later shown to owe its profound cancer-inducing phenotype to carrying the K-ras oncogene. Indeed, I first learned about the whole class of “oncogenes” and the fact that they are really altered derivatives of genes that regulate normal cell growth from the work of two prior awardees, Mike Bishop and Harold Varmus.
Armed with this background, I began probing DNA, RNA and proteins from various human cancer tissues removed therapeutically, looking for alterations in these genes and publishing our first results in 1984. This initial work led to the pivotal 1986 collaboration with Axel Ullrich who had identified six growth-related genes, including HER2. We were agnostic in our initial approach to studying these genes and evaluated a large series of different cancers for changes in them without much success until we came to breast cancer tissues. It was here we first saw that HER2 was amplified in about 20-25% of the cases and developed the initial evidence that HER2 amplification identified a new subtype of breast cancer; those with the worst outcomes despite being treated using our best available standard therapies. By then, Bob Weinberg had shown that the murine counterpart of HER2, when mutated, would cause brain malignancies in rats and Jeff Drebin subsequently demonstrated that antibodies directed against the protein made by this mutated murine-HER2 version would inhibit growth of mouse tumors. Again learning from colleagues, Mike and Axel at Genentech and we at UCLA undertook preclinical testing of a series of HER2 antibodies and saw similar growth inhibition of HER2-driven cell lines and experimental tumors. This preclinical work culminated in the subsequent clinical development and eventual approval of the drug Herceptin that has now changed the natural history of patients with HER2-positive breast cancer from those with the worst outcomes to those with the best.
Finally, the three of us and more than 3 million women globally, also stand on the shoulders of a small group of women who volunteered to participate in the initial clinical trials ultimately resulting in approval of Herceptin. They are not research subjects or study numbers but are also colleagues in every sense of the word. We owe them a profound gratitude as well. The lessons learned on all these shoulders; those of colleagues and patients, as well as the platforms we initially built to study HER2, continue to inform us today and recently led to research resulting in approval of agents targeting the cdk-4 and 6 kinases and producing major outcome improvements for hormone receptor-positive breast cancer, the largest subtype of this disease.
I have had the empowering advantage of standing on the shoulders of others including my co-awardees and I thank all of those many individuals and you for this amazing honor.
Acceptance remarks, 2019 Lasker Awards Ceremony
More than a hundred years ago, Paul Ehrlich, the founder of chemotherapy, received the Nobel Prize for Physiology or Medicine. He postulated ‘man muß zielen lernen, chemisch zielen lernen’ (you have to learn targeting, chemically targeting) – to find ‘magic bullets’ – chemical substances that might have special affinities for cancer cells in the fight against human diseases. This concept inspired generations of scientists, including me.
Since I was a boy, I had developed an interest in everything that lived – plants and animals. After studying biochemistry in Tübingen, I went on to do my PhD in Heidelberg. I was always interested in the application of science and I wanted to do something medically important. I had read about the newly emerging gene technologies in the mid-seventies and was very excited. UCSF was a cosmos of creativity. I started to work on insulin, on cloning insulin cDNA, and I succeeded. From then on, my research and my career developed along a path that really started with basic scientific curiosity, but in a field that was very close to application. That my work led to cancer research was not planned either. This came out of my interest in growth factors and their receptors, and suddenly there was this connection to cancer. The discovery of HER2/neu, it’s role in breast cancer and the development of Herceptin – ‘the magic bullet’ – all resulted from that interest. However, as is common since long, all this work was a great team effort.
At the time of oncogene discoveries and in the early years of targeted therapy I was optimistic that cancer will be curable one day. I think you have to be a little bit naive to tackle large problems such as cancer. Today, I am more realistic – there is still a long way to cure cancer.
To receive the Lasker award is a great honor. I feel very grateful that so many patients with cancer have benefitted from our work – this is a dream come true for a scientist.