Douglas Coleman
Jackson Laboratory

Jeffrey M. Friedman
The Rockefeller University
For the discovery of leptin, a hormone that regulates appetite and body weight — a breakthrough that opened obesity research to molecular exploration.
The 2010 Albert Lasker Basic Medical Research Award honors two scientists for their discovery of leptin, a hormone that regulates appetite and body weight. Douglas Coleman (Jackson Laboratory) established that an appetite-suppressing substance circulates in the bloodstream and signals a second molecule to curb hunger. Jeffrey M. Friedman (Rockefeller University) isolated the gene that encodes the appetite suppressant and showed that fat cells release it. Their studies and subsequent findings demonstrated that this chemical, leptin, plays the central role in a self-regulating circuit: As fat accumulates, it exudes leptin, which binds to a receptor in the brain that quells the desire to eat. Coleman and Friedman have launched new understandings of obesity and disorders that result from perturbed leptin activity. They have overturned conventional notions with the insight that many overweight people suffer not from lack of willpower, but from metabolic disruptions.
Individual humans eat approximately a million calories per year, yet most people's weight remains stable over long periods. By the 1950s, scientists were devising schemes for how the body balances food intake with energy expenditure. In 1953, Gordon C. Kennedy (National Institute for Medical Research, London) suggested that some compound, whose quantity mirrors that of stored fat, acts on the brain's hypothalamus, a site that was known to house appetite centers. In the late 1950s, Romaine Hervey (University of Cambridge) created lesions in the hypothalamus that subsequently caused rats to eat voraciously. He then surgically connected pairs of animals — one with hypothalamic damage and one with an untouched brain — such that elements of their circulatory systems mixed. The normal rats stopped eating, Hervey reported. He proposed that chemical information from an overfed body travels through the bloodstream to intact appetite centers, which respond by subduing hunger in the normal animals.
Award presentation by Michael Brown
 This year's Lasker Basic Science Award honors two scientists who challenged our notion of free will. Let me explain. The thin among you take pride in your willpower. Through rigid self-control, you pass up rich deserts and you never touch a French-fried potato or a pepperoni pizza. Only the slothful succumb. Today's honorees taught us that you shouldn't be so smug. Your willpower alone doesn't protect you from gluttony. It needs help from your leptin.
This year's Lasker Basic Science Award honors two scientists who challenged our notion of free will. Let me explain. The thin among you take pride in your willpower. Through rigid self-control, you pass up rich deserts and you never touch a French-fried potato or a pepperoni pizza. Only the slothful succumb. Today's honorees taught us that you shouldn't be so smug. Your willpower alone doesn't protect you from gluttony. It needs help from your leptin.
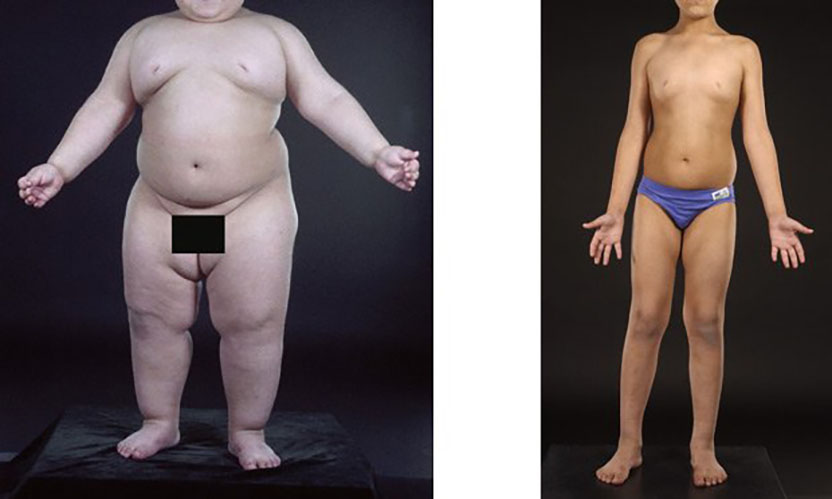
Let me illustrate. Each of you received a booklet describing today's ceremonies. Please turn to page 15. At the top you see a three-year-old baby who weighs 92 pounds. Imagine hefting a 92-pound baby around the zoo. The baby is fat because he never stops eating. His body cannot produce a hormone called leptin. His two genetic templates for leptin are both defective. Leptin is a hormone that circulates in the blood and enters the brain, where it makes us stop eating. If our bodies lack leptin, we are condemned to eat ourselves to death, willpower or no.
Now look at the picture on the bottom. This is the same boy at age 7. His parents have injected him daily with leptin. His appetite is tamed and he has joined the ranks of the strong-willed. Not because of his will, but because of his leptin. The lesson is clear. If any of you were deficient in leptin you would weigh 400 pounds. Right now you would be begging for a second helping of desert. So much for free will.
Acceptance remarks
Acceptance remarks, 2010 Lasker Awards Ceremony
I am overwhelmed and humbled to be selected as a co-winner of the 2010 Lasker Basic Medical Research Award. I have always viewed the Lasker as one of the most esteemed biomedical awards, and it is with great pride that I accept this honor.
As the only son of parents who had little formal education, I was encouraged to excel in school. During undergraduate studies at McMaster University, a very dynamic professor taught me the rudiments of biochemistry and an appreciation of the scientific method. With his enthusiastic support, I entered graduate school at the University of Wisconsin and received my PhD in 1958. Rather than starting postdoctoral studies or a career in academia or industry, I became a research scientist at The Jackson Laboratory. This decision was transformative: The Jackson Laboratory had fascinating genetic disease models, interactive colleagues, and Acadia National Park as a backyard.
Initially, I had no intention of studying the diabetes/obesity syndrome, but in 1965 a spontaneous mouse mutation was discovered and I began research that would consume much of my scientific thought for the better part of three decades. This new mutant was similar to a previously characterized obese mutant, except that it displayed severe life-shortening diabetes. Operating on the hypothesis that a blood-borne factor might regulate the severity of diabetes in these mutants, I used a technique called parabiosis to mix and match mutant and wild-type blood supplies. Based on these experiments, I concluded that the diabetes mutant overproduced, but did not respond to, a blood-borne 'satiety factor', while the obese mutant recognized this factor but was unable to produce it. Subsequent studies suggested that adipocytes synthesized this factor and the hypothalamus contained its receptor. The scientific community did not readily accept these conclusions because obesity was considered strictly a behavioral problem, not a physiological problem.
Definitive proof of my conclusions required isolating the satiety factor — a feat that resisted rigorous experimentation. Finally, in 1994 Jeffrey Friedman succeeded in identifying the obese gene and demonstrated that it encoded the powerful hormone leptin. With the subsequent cloning of the leptin receptor, the field exploded. Essentially all of my predictions were verified: the obese mutant lacks active leptin, which is normally produced in adipose tissue; the diabetes mutant lacks an active leptin receptor, which is normally expressed in the hypothalamus. With these findings, two long-standing misconceptions were definitively laid to rest: obesity was not merely a behavioral problem but rather had a significant physiological component, and adipose tissue was not merely a fat-storage site but rather an important endocrine organ.
In closing, special thanks go to my family who were not only supportive of my work but a welcome diversion from it. My wife for nearly 55 years, Beverly, merits my strongest gratitude because she, more than anyone, built the love, support, and understanding that was the foundation on which I functioned. She would have been delighted to share in the accolades from this most prestigious award.

Acceptance remarks, 2010 Lasker Awards Ceremony
It is a profound honor to receive this year's Lasker Award for Basic Research. The significance of this prize is heightened by the ground-breaking achievements of the prior recipients, who are among the greatest contributors to medical science, and the depth of the esteem in which each of the committee members is held. I would also like to thank the many people who have helped me, and a list of these people's names are included in my full acknowledgements in the Lasker brochure.
In 1903, Theodore Roosevelt wrote, "Far and away the best prize that life offers is the chance to work hard at work worth doing." While there is wisdom in this statement, I have come to conclude that it fails to capture that which motivates me. For me the best prize was having had the chance to make a discovery.
As scientists we are the instruments of a wave of new knowledge that passes through of us from the past and into the future. The wave of knowledge of which my research is a part began with the realization of the French chemist Lavoisier in the 18th century that living organisms are subject to the basic laws of physics and chemistry, the formulation of the first law of thermodynamics by Joule and Meyer, passes through Hetherington and Ranson, two great neuro-anatomists, and Gordon Kennedy and Romaine Hervey, two physiologists of the 20th century who suggested that fat-tissue-generated signals that regulated body weight. In a beautiful set of experiments for which he is being recognized, Doug Coleman of The Jackson Laboratory correctly predicted that this factor was encoded by the mouse ob gene.
This wave of new knowledge coursed briefly through me, highlighted by a singular moment when the presence and absence of a few regions of intensity on an X-ray revealed the answer to a simple question that had perplexed scientists since Lavoisier: How do biologic systems count calories? Or more precisely, how does Nature monitor the number of calories stored by fat? We now know that this is achieved by the production of a hormone by fat tissue, and it was the realization that the amount of body fat is regulated by this hormone, leptin, that led me to be recognized today. This moment of discovery was exhilarating. Gazing upon the X-ray was also humbling because it revealed the beauty and power and majesty of Nature, which had solved the problem of counting calories so elegantly and simply. The only moments in my life that compared to this were when I was married and when I heard my twin daughters cry for the first time as they were delivered.
This wave of knowledge that began with Laviosier and passed through Doug, then me, will continue into a future that I cannot clearly see but which others undoubtedly can or will. This future puts forth the promise that in time we will understand how complex behaviors such as feeding are regulated at the cellular and the molecular level.
The realization that leptin and other molecules control feeding behavior and body weight show that obesity is not a personal choice. It is my hope and expectation that the realization that obesity has a biologic basis will not only lead to new treatments but also lead to a greater sense of understanding for the obese. It is simplistic to imagine that we can consciously control all of our basic drives, drives that have been honed by evolution for countless millennia.
As a child, it would have been inconceivable to me that I would ever have my name listed with the previous recipients of this award, and it is the deepest of honors. The depth of this occasion is completed by the presence of my friends, colleagues, and family, in particular my wife, Lily, and my daughters, Nathalie and Alexandra.
Interview with Doug Coleman and Jeffrey Friedman
Video Credit: Susan Hadary