Alain Carpentier
Hôpital Europeen Georges Pompidou

Albert Starr
Providence Health and Services
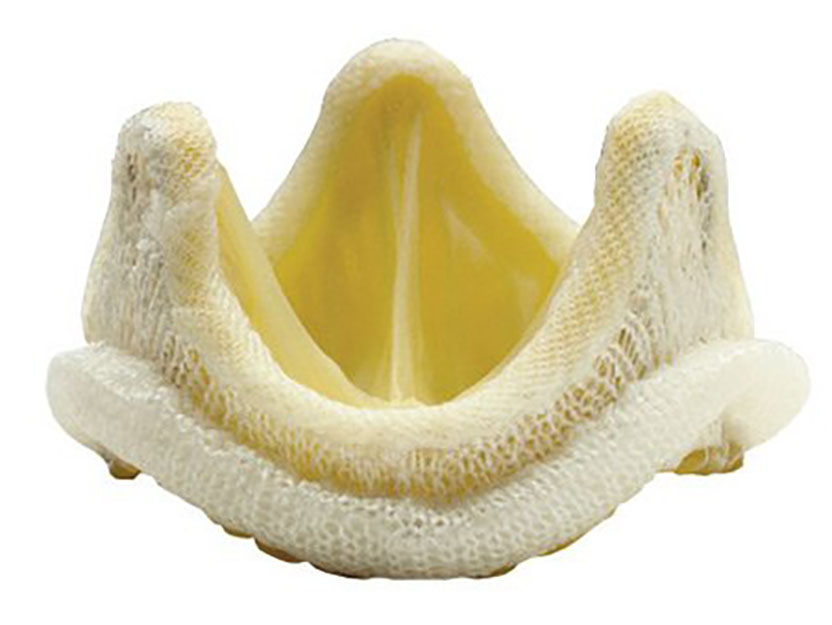
For the development of prosthetic mitral and aortic valves, which have prolonged and enhanced the lives of millions of people with heart disease.
The 2007 Albert Lasker Award for Clinical Medical Research honors two surgeon-scientists who revolutionized the treatment of heart disease. Albert Starr and his engineer partner, the late Lowell Edwards, invented the world’s first successful artificial heart valve. This device has transformed life for people with serious valve disease, providing a remedy where none previously existed. Alain Carpentier then circumvented the predominant limitation of mechanical valves — a propensity to clot within blood vessels and the associated need to take blood thinners — by adapting animal valves for use in humans. In the embryonic days of open-heart surgery, Starr and Carpentier opened up the entire field of valve replacement. Their work has restored health and longevity to millions of individuals with heart disease.
Starr’s and Carpentier’s contributions extend beyond these landmark innovations. In an era before the US Food and Drug Administration (FDA) regulated medical devices, Starr set up the infrastructure for conducting clinical trials on his valves, including an informed-consent procedure and long-term patient tracking. This practice allowed him to evaluate valve-replacement outcomes and seek solutions to clinical problems. Furthermore, his surgical patients required a new type of postoperative care. To deliver it, he assembled a multidisciplinary healthcare team, creating what corresponds to today’s cardiac intensive care unit. Carpentier, in turn, augmented his own initial discovery by formulating techniques to repair rather than replace valves — a venture that was aided by the availability of prosthetic valves as a backup. He continues to probe the suboptimal areas of heart-valve surgery, relentlessly pursuing superior strategies.
Award presentation by Michael Brown
If you’ve ever used a hand-held pump to inflate a bicycle tire, you know that pumps need two valves — one to permit air to enter on the intake stroke, and the other to permit air to exit during expulsion. Our hearts are no different. Each of the two pumping chambers has two valves — one for intake and one for expulsion. Heart valves are remarkably resilient. Over a human lifetime they open and close 3 billion times, allowing 50 million gallons of blood to pass. When they close, they prevent the backflow of this deluge. It’s no wonder that heart valves wear out. They can wear simply with age, or they can fail early in life as a result of birth defects, or diseases like rheumatic fever and bacterial infections. Disease can constrict the valve, blocking the flow of blood, or it can render the valve leaky, permitting devastating back-flow. Often a single valve can suffer both problems.
Acceptance remarks
Acceptance remarks, 2007 Lasker Awards Ceremony
Progress in medicine is often triggered by emotional circumstances. As a resident in cardiac surgery in the early 1960s at the Hôpital Broussais in Paris, I was struck by an artist suffering from a valvular disease whose valve had been replaced by a valvular prosthesis. Three months after the operation, he presented with a severe brain damage due to the migration of a clot formed in the contact of the prosthesis. He couldn’t paint anymore. The same valve, which had saved his life, had now definitely impaired his quality of life. At this very moment, I decided to devote my research to this problem.
But how to start? Which direction to take? I knew that valves removed from human bodies and grafted to a patient did not produce clot formation. Unfortunately, this solution was complex in its implementation because of problems of procurement and infection. Valves retrieved from animals shouldn’t have these disadvantages. Unfortunately, pig valves implanted in sheep were destroyed in a few days or weeks by acute immunological response. Something had to be done to prevent it. This required a much better expertise in immunology and chemistry than the one I had acquired during my medical education.
Although I was already an active cardiac surgeon, I persuaded with difficulty my chief, Professor Dubost, that I should spend one or two days a week at the Faculty of Sciences. At the same time, I developed my own research laboratory, actually a single small room in the basement of a building of my faculty. Exploring all chemical techniques of tissue fixation, I was fortunate to discover that glutaraldehyde could reduce the immunological response enough for the valve not to be rejected.
In research, more challenging than an idea itself, application requires favorable circumstances and some luck. I was lucky in 1969 to receive the visit, in my laboratory in Paris, of the famous surgeon Albert Starr. He was so surprised by my results that he advised me to develop this new valve in collaboration with the laboratory which was already manufacturing his own prosthesis. Using the engineering capacities of Edwards laboratories, the glutaraldehyde-treated pig valve was mounted into a stent to facilitate its surgical implantation in a human. I called it “bioprosthesis” to refer to its biological origin and its prosthetic fate.
The first implantation of a valvular bioprosthesis took place in May 1968 in Paris during one of the most famous revolutions of students. The operation succeeded, the revolution faded. Several other successful operations followed with the advantage for the patients not to be obliged to take any blood thinner. A 25-year-old marathon runner wanted an additional advantage: “I want a bioprosthesis,” he told me, “but can’t you make it from antelope tissues?” — Why? — “Because antelopes run faster than pigs!”
The clinical results had been gratifying for six years when an unexpected complication emerged in patients younger than 60 years of age: tissue calcification that compromised long-term valve function. I returned to the lab and, with the help of my wife, Sophie, I improved the method of glutaraldehyde fixation by adding calcium-mitigating adjuncts. In the same time, I improved the valve design to minimize flow turbulence, an additional cause of calcification. These improvements almost doubled the durability of the bioprosthesis and extended the indications to younger patients. A lot of research work, however, remains to be done in order for this valve to be used in children. This is what I am doing now.
This prestigious award is the highlight of my career and a strong incentive to persevere, thus continuing to follow the St. Augustine’s motto, which has influenced my whole spiritual and scientific life: “Always search, and when you have found, search again.” Merci beaucoup to the Lasker Foundation and to its Jury.

Acceptance remarks, 2007 Lasker Awards Ceremony
I feel honored to accept this prestigious award and recognition by the Lasker committee. This occasion brings to mind three stories about our work. The first concerns Lowell Edwards, my engineer partner; the second, a dilemma in going from animal to man; and the third, a call from a grateful patient 43 years later.
Edwards and I began work in 1958, and early on he was interested in obtaining a unit of human blood. I supplied it with some difficulty and wondered why he needed it. The next day I visited his laboratory in a wood shed at his home on the Sandy River and he had the blood in a device that I had never seen.
He was measuring its lubricity; the S.A.E. of blood! No one had ever done so to his knowledge, and this could be an important element in valve durability. This consideration would drive valve design so as not to extend the capabilities of blood as a lubricant. This was a stunning moment as I realized the importance of the interface between medicine and engineering. This interface would grow enormously in the next few years with multiple technology companies devoted to cardiac surgery. Edwards Laboratories, now Edwards Lifesciences, became the prototype for a massive industrial complex supporting medicine.
The second story involved the problem of animal to man in medical research. After implanting many valve types in dogs, all of which thrombosed in a few days, we eventually used a ball and cage device. Unfortunately, all animals died of valve thrombosis at about 30 days after implantation, except for one beautiful black Labrador retriever. We then developed a complex mechanism to shield the zone of implantation, with 80% long-term survivors beyond 6 months. As a result of the success of the shielded valve, we were urged by our chief of Cardiology in General Surgery to initiate clinical trials, but which valve should we use—the simple unshielded valve with our one survivor, or the shielded valve that was truly successful in the experimental animal? We chose the unshielded valve—it was the right choice, and successful in man. If we had chosen the complex shielded device, and if it was successful, how would we know if the unshielded and simpler device would also have been successful? On the other hand, if the unshielded valve failed, we could always move to the shielded valve. This decision was a good one and I owe a debt of gratitude to a beautiful dog who was adopted as a mascot by our laboratory team.
Finally, the third story involves a patient I operated on in Salonika, Greece, in 1964. The chest was opened, the patient was on bypass, her own destroyed valve was removed, and I asked the nurse for a 30-mm ball valve. The valves had been delivered to her in little plastic containers to be autoclaved. There was hesitation, then tears as she told me in broken English that the valves we brought to the operating room were destroyed in the autoclave. Apparently she had sterilized the valves in their little plastic bags, the plastic melted and was adherent to the valve. It looked as if the valve had melted. In fact, it was only the plastic bag, and it was possible to peel this off the device and implant it successfully. Two months ago on a visit to Athens, Greece, I got a thank you call from Salonika, and it was this patient who was alive and well with the same device 43 years later. As we spoke I had vivid recall of her operation. This was but one of hundreds of calls and letters received from grateful patients, providing much positive feedback for our work. Recognition by the Lasker Foundation has added a meaningful new dimension for what has been a very happy story.