For many people, summer offers a chance to lounge at the beach, visit relatives, or hike in national parks. Albert Coons spent his time in the summer of 1939 observing autopsies and thinking about diseases such as leukemia and rheumatic fever, a common complication of bacterial infections at the time. The 27-year-old, who had just finished a two-year stint as house officer at Massachusetts General Hospital, was waiting to start a job as an assistant resident at Boston City Hospital. With six months off, he had traveled to Berlin, where a friend worked as a pathologist at a local hospital.
The two got together often to discuss science and medicine. Coons also had plenty of time for “reading or brooding,” he recalled. One day he began musing about the microscopic nodules that grow in the hearts of rheumatic fever patients. Doctors suspected that the nodules formed when an unidentified bacterial molecule sparked an immune response. To test the hypothesis, however, they needed to confirm that the inciting molecule, known as an antigen, was lurking in the lesions. Coons, who had studied antibodies during his medical training, hit on the idea of using an antibody tagged with a colored molecule to highlight the antigen.
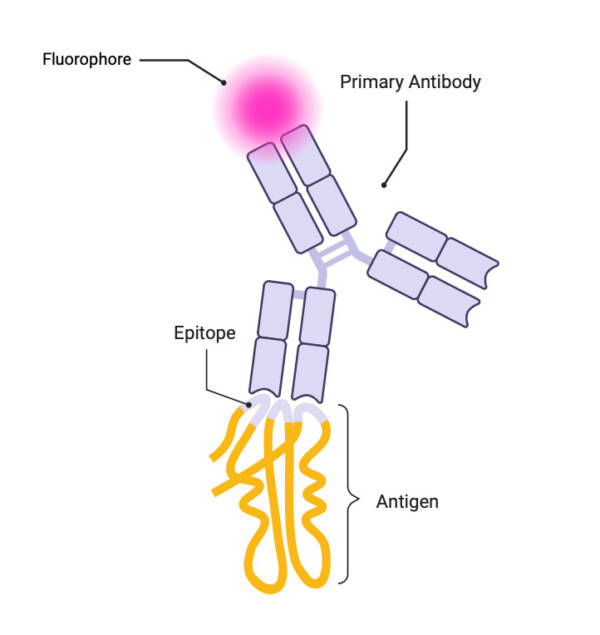
Schematic of the basic concept of primary immunofluorescence
Courtesy of Mirna Jarovic
Coons ran the notion past his pathologist friend, who dismissed it. “I think he thought it was not feasible,” Coons recalled. But the revelation spawned a technique known as immunofluorescence that became a diagnostic and research mainstay and earned Coons, then at Harvard Medical School, the 1959 Albert Lasker Basic Medical Research Award. Immunofluorescence involves attaching fluorescent compounds to antibodies to create molecular homing beacons. The antibodies latch onto an antigen, usually a protein, and signal its presence in a cell, tissue, or organ. “We use it in the lab every day,” says Steven Hrycaj, technical director of the immunohistology laboratory at the University of Michigan Medical School, which analyzes about 150,000 patient samples per year. “It saves lives.”
The basics of immunofluorescence have changed little, Hrycaj says. “What Coons did is almost exactly the same as what we do nowadays.” But in recent years, researchers also have devised new applications for the technique and expanded its capabilities. Surgeons have enlisted immunofluorescence to delineate the boundaries of tumors and make them easier to remove, for instance. Instead of a single color, modern versions of immunofluorescence offer a palette, allowing detection of many proteins. “We can do 60 different antibodies on a single sample,” says surgical pathologist Aatur Singhi of the University of Pittsburgh. And scientists are working to develop even more uses for Coons’s brainchild.
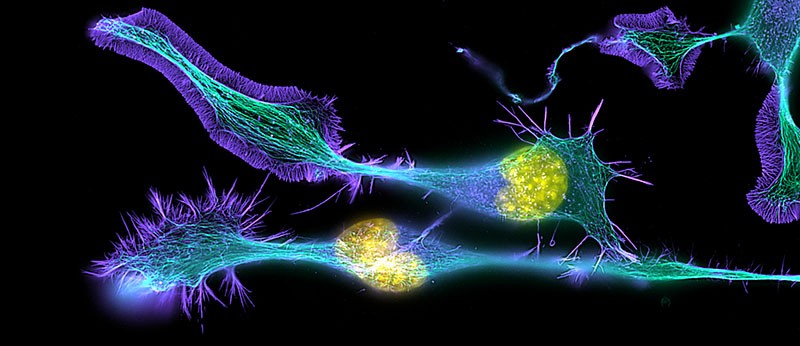
Immunofluorescence photomicrograph of cultured cells from a neuroblastoma. Fluorescent dyes highlight proteins in the cell nuclei (yellow) as well as cytoskeletal protein filaments tubulin (green) and actin (blue).
Courtesy of Torsten Wittmann / Science Source
Coons said that his insight was “perfectly obvious.” But he was underselling his accomplishment, according to immunologist Hugh McDevitt of Stanford University, who wrote an appreciation of Coons’s career. Given how little scientists knew about antibodies in 1939, “the concept of putting a visible label on an antibody molecule seems both bold and original,” McDevitt wrote.
Still, turning that concept into a usable lab technique proved difficult, as Coons learned when he returned to the United States later in 1939. For one thing, his clinical work claimed most of his time. On the rare occasions when Coons did get into the lab, his attempts to link colored molecules to antibodies failed. He started making progress after taking a research fellowship that freed him from clinical duties. Coons managed to affix colored molecules to antibodies and use them to mark bacteria. But the results were disappointing because “under the microscope the organisms were only faintly pink,” he recalled. “There was no hope of being able to find small amounts of antigens in tissues with color of this intensity.”

First page of the article describing Coons’s method
Looking to brighten his antibodies, Coons contacted an organic chemist at Harvard, who suggested that he “‘go downstairs and talk to two fellows in the basement who are already hooking fluorescent compounds to proteins.’” Coons teamed up with the two researchers to attach a molecule that fluoresces blue under UV light to antibodies that recognize an antigen on the surface of some bacteria. When the scientists added the labeled antibodies to bacterial samples, the microbes were “brilliantly fluorescent under ultraviolet light,” Coons wrote. A 1941 paper presented their results.
But that was only the first step. Coons wanted to detect antigens in samples from patients. Yet under UV light, the tissues naturally produced a bluish glow that obscured the signal from the labeled antibodies. To circumvent that problem, he and his colleagues switched to a new tag—a compound that fluoresced green and would stand out against the body’s natural blue—also enlisting a Harvard chemistry graduate student to synthesize it. After equipping their antibodies with the molecule, the researchers showed that they could pinpoint bacteria in mouse tissues.
When they achieved that breakthrough, the United States had entered World War II. Coons soon joined the military as a doctor. He wrote the paper describing the team’s findings while taking a train across the country so that he could ship out for duty in the South Pacific. A colleague submitted the work to a journal, but Coons admitted that he lost track of it until 1943, when copies of the journal “reached me at Brisbane in Australia as I was boarding a ship to go north to New Guinea.”
Coons served in the US Army for four years as a pathologist and as director of hospital laboratory services. Returning to Harvard in 1946, he wanted to resume his research on immunofluorescence. The previous experiments’ reagents were still in a refrigerator on campus, but because none of his chemistry colleagues wanted to make the fluorescent labels he needed, he had to learn to synthesize them himself. Collaborating with different researchers, Coons fine-tuned the technique and showed its powers in studies published in 1950 and 1951. The team showed, for instance, that immunofluorescence could detect viruses in mice. Coons soon shifted to investigating how the body makes antibodies.
In later years, immunofluorescence’s specificity, resolution, and simplicity earned it a place in diagnostic labs around the world. Hrycaj says that his facility performs the technique on about 20 specimens per day. Immunofluorescence isn’t the only option for making diagnoses from tissue samples, he says. Pathologists often prefer a similar technique called immunohistochemistry—which involves labeling antigens with antibodies linked to nonfluorescent molecules—because it’s faster and cheaper. With immunohistochemistry, only a standard bright-field microscope is necessary to view slides of tissue samples, whereas immunofluorescence requires costlier fluorescence or confocal microscopes. Nonetheless, immunofluorescence is the go-to method for diagnosing conditions such as certain kidney and skin diseases. “When we are looking for something that is very sensitive and where we have to quantify, that is where immunofluorescence shines,” Singhi says. Still, immunofluorescence’s higher cost and requirement for specialized equipment deters some clinics from using it, he says.
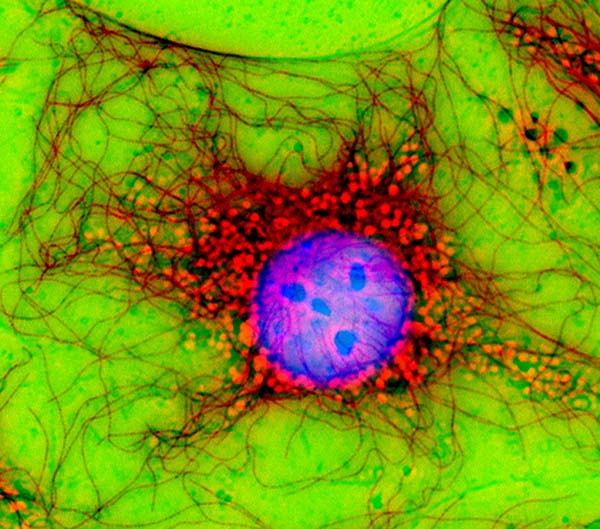
Immunofluorescence photomicrograph of a fibroblast cell; the cell nucleus is purple, microtubules are red.
Courtesy of Jan Schmoranzer / Science Source
Recent advances have boosted immunofluorescence’s diagnostic value. Coons and colleagues tested for one antigen at a time. A new variety called multiplex immunofluorescence, introduced in the last decade, can simultaneously pinpoint many antigens by using different antibodies, each sporting a unique color. That capability is important for patients, Singhi says, because pathologists can extract more information from small tissue samples. “The future of immunofluorescence is multiplexing,” he says.
Scientists also have adopted immunofluorescence in their work for many of the same reasons as clinicians. “In research, it’s the standard,” Hrycaj says. The technique is illuminating because it can pin down where particular proteins reside, how abundant they are, and how they interact—key information for cell biologists, neuroscientists, immunologists, cancer biologists, and other researchers. Unlike two-dimensional immunohistochemistry, immunofluorescence can enable researchers to analyze protein distribution in three dimensions, giving them a clearer picture of tissues’ organization and function.
Researchers such as Eben Rosenthal of Vanderbilt University Medical Center have developed new applications for immunofluorescence. “We have taken it to the next logical conclusion, which is to administer it to patients,” says Rosenthal, also a head and neck surgeon. About 20 years ago, Rosenthal and his team began testing whether injected fluorescent antibodies could aid surgeons by marking the edges of tumors, which are often hard to distinguish from healthy tissue. Those studies and research by other scientists indicated that the technique may be beneficial. With immunofluorescence, “you can identify cancer in real time in the operating room,” he says, and some surgeons use the technique to guide their scalpels.
Rosenthal and colleagues are now trying to determine whether immunofluorescence can help doctors choose patients to receive immune checkpoint inhibitors and to fine-tune dosages of those antibody-based cancer treatments. Checkpoint inhibitors first gained FDA approval more than a decade ago and earned James Allison of the University of Texas MD Anderson Cancer Center the 2015 Lasker~DeBakey Clinical Medical Research Award and a share of the 2018 Nobel Prize in physiology or medicine. Researchers still haven’t figured out optimal doses for the treatments, Rosenthal says. “What’s amazing is that we think of this as precision medicine, but we give the same dose of antibodies to everyone.” Identifying the most effective dosage is all the more important, he says, because the drugs cost about $10,000 per dose.

Coons (1959)
Although checkpoint inhibitors work against more than 20 cancers and can cure some patients, only about 20% of recipients benefit from the drugs. Rosenthal and colleagues hope to boost that number. Their previous research with fluorescently labeled antibodies suggested that the drugs often don’t spread throughout tumors. “There are dead zones where the drug doesn’t reach,” he says. This year the researchers launched a clinical trial that may indicate whether modifying doses of the drugs can permit more even dispersal. The participants, all with head or neck cancers, will receive different doses of the checkpoint inhibitor panitumumab as well as a modified version of the fluorescently labeled antibody. After removing the tumors surgically, Rosenthal and colleagues will measure the fluorescence in the samples to determine how much panitumumab penetrated cancerous tissue, allowing them to deduce the most potent dosage. Patients today receive the highest dose they can withstand, Rosenthal says, but the drug “may work at one-half or one-third of the maximally tolerated dose,” potentially reducing side effects and boosting the treatment’s effectiveness.
Coons was shy, McDevitt noted, and “remained a little amazed and perhaps a bit embarrassed at how widely successful his method became.” Coons wrote that he could hatch his signature idea because he’d done plenty of intellectual preparation and because he had spare time to “give the subconscious a chance.” The path to a creative breakthrough, he wrote, was “First, the hard stocking of the mind with the facts and the struggle over them; then the latent period; and finally, the sudden insight into the solution.”
By Mitchell Leslie

