Q: One of the many uses of the in situ hybridization technique that you developed is to diagnose chromosomal integrity. What other medical insights have ensued from your research?
A: In situ hybridization has indeed become an indispensable tool in cell biology. The original technique that I developed together with my student Mary Lou Pardue used radioactive RNA or DNA probes. This technique was later replaced by much more convenient fluorescent probes, detected by fluorescence microscopy. Fluorescent in situ hybridization (FISH) is now used in myriad diagnostic procedures to detect chromosomal abnormalities and to localize RNA molecules in the cell.
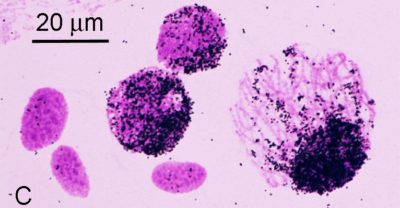
Autoradiographs of nuclei from the overlay of the toad Xenopus, after in situ hybridization with radioactive ribosomal RNA. Three unlabeled follicle nuclei (smaller, almond shaped) and three labeled pachytene nuclei. Image credit: Joseph Gall
Another area of my research that had unexpected consequences was the discovery of the telomere sequence in 1978. I was studying the genes coding for ribosomal RNA (rDNA) in frogs and other organisms. During formation of the egg, these genes somehow get out of the chromosomes and replicate independently. I isolated the rDNA of the [single-cell organism] Tetrahymena, which has a giant nucleus. A new postdoc in my lab, Elizabeth Blackburn, had just come from Fred Sanger's lab at [the University of] Cambridge and knew how to sequence DNA — or at least a few nucleotides of DNA. We decided to sequence the ends of the rDNA molecules of the Tetrahymena, and they turned out to have a simple sequence, CCCCAA, repeated over and over. We did not know it at the time, but the same or similar hexanucleotide sequences occur at the ends of chromosomes of nearly all eukaryotic organisms. These so-called telomere sequences are important for the integrity of chromosomes.
Q: What is the secret to your ability to choose experimental materials and species that are uniquely suited to answer the questions you are asking?
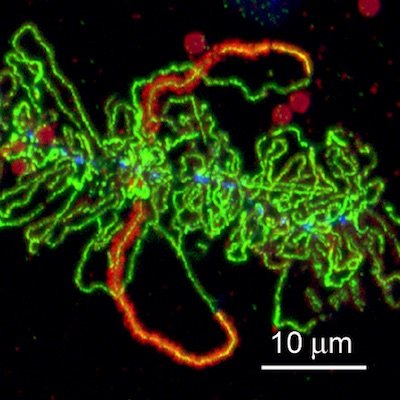
Lampbrush chromosome, immunostained to show RNA polymerase (green) and an alternative splicing factor (orange). Image credit: Joseph Gall
A: Long before I became a cell biologist and molecular biologist, I was a biologist interested in natural history. From an early age, I spent many hours collecting butterflies and moths, examining pond organisms with my microscope, and rearing all kinds of insects and other creatures. So I had direct experience with a variety of plants and animals, and I knew what characteristics might be useful for a particular study.
Another advantage was that I had read the 'bible' of cell biology, E.B. Wilson's The Cell in Development and Inheritance. In this book, Wilson cites many examples from the classical literature of the 19th and early 20th centuries. Knowing about this earlier work gave context to some of my molecular studies.
Q: What has been your most exciting discovery in the past 5 years?
A: Probably our most unexpected finding during the past few years has been the discovery of stable circular RNAs derived from introns [noncoding sections of an RNA transcript, or the DNA encoding it], both in the nucleus and in the cytoplasm. They are interesting because they are so widespread, occurring in all cell types we have examined so far. The function of these intronic sequences is not at all obvious and will be a challenge to determine. As I like to remind my colleagues, introns themselves were discovered 40 years ago, yet we still have no overall theory to explain their existence, let alone their function in a mechanistic sense. There are several powerful new techniques that can now be applied to elucidate the structure and composition of these transcription units, including deep sequencing, analysis of chromatin looping, and super-resolution microscopy.
Q: Who were the mentors who had the biggest impact on your success?
A: There is no question that my most important mentor was my mother. She was the first in her family to attend college, matriculating at Goucher College in Baltimore in 1920. She majored in mathematics, which was remarkable for a woman at that time. In the days between the two World Wars, there were essentially no professional opportunities for a woman in science. Instead, my mother encouraged my interest in nature and science from an early age. She made numerous butterfly nets for me and helped me with my insect collection. Before I was in high school, she and my father got me a genuine research microscope and even a microtome. I examined all sorts of protozoa and other microscopic organisms as living specimens; I embedded tissues, cut thin sections, and stained them with a variety of classical stains. My mother made sure I had lots of books. I had a copy of E. B. Wilson's The Cell in Development and Inheritance, and I was fascinated by the many wonderful things described by this pioneer in cell biology.
My PhD advisor at Yale, Donald Poulson, was a Drosophila geneticist who had worked with Alfred Sturtevant and other greats in this field at Cal Tech. He was completely nondirective as an advisor and let me work on a topic unrelated to his primary interest. Instead of Drosophila, my thesis was in the field of the giant 'lampbrush' chromosomes found in oocytes of frogs and salamanders. His hands-off style of mentoring suited me perfectly and fostered my independence.
I have also been mentored throughout my career by my many scientific colleagues, collaborators, and especially the students with whom I have shared the joys of discovery.
