The bad news arrived when Peter Ratcliffe was just three weeks into his MD research program. The scientist at the University of Cambridge who was supposed to supervise his work suddenly decided to leave. Because the MD degree in the UK system is akin to the PhD in the United States, the professor's move stranded Ratcliffe without scientific guidance, a lab where he could conduct experiments, and money to pay for them. The departing researcher "had a party at his house with cake," Ratcliffe recalls. "But I was desperate," he says. "This was a time when I could easily have gone down."
Instead of sinking, however, Ratcliffe took charge. He designed his own experiments and found lab space. With help from colleagues, he rounded up enough grant money to support his research and pay a lab technician. Ratcliffe knew when to ask for a hand, says his longtime friend and collaborator Chris Pugh, a nephrologist at the University of Oxford, but "he was essentially self-supervised and self-trained."

Ratcliffe, shown here in 2019 at the Ludwig Institute for Cancer Research, continues to study how cells sense oxygen.
Courtesy of Ludwig Institute for Cancer Research
That Ratcliffe forged ahead with his MD research—he completed the degree in 1987—illustrates one of the main reasons for his scientific success, Pugh says. "He's dogged. He doesn't give up on the important things." That determination served him well when he began investigating a seemingly unpromising topic: how cells sense oxygen levels. Ratcliffe's discoveries earned him the 2016 Albert Lasker Basic Medical Research Award and the 2019 Nobel Prize in physiology or medicine. He shared both awards with two scientists who helped untangle the oxygen-sensing mechanism: cancer biologist William Kaelin of Harvard Medical School and medical geneticist Gregg Semenza of Johns Hopkins University.
The mechanism's discovery was a milestone because "oxygen is one of the fundamental necessities of life," says cell and molecular biologist Sonia Rocha of the University of Liverpool, who had no role in Ratcliffe's research. To carry out metabolism, cells need oxygen, and its availability influences an array of other cell functions, such as which genes are active. Since the 1800s, scientists had been trying to understand how cells sense oxygen and respond appropriately, she says. Ratcliffe stands out not just because of what he discovered but also because of how he discovered it, says molecular biologist Randall Johnson of the Karolinska Institute in Sweden, who also wasn't connected to Ratcliffe's research. "His work is marked by a high degree of rigor and attention to detail." At the same time, Johnson says, "he's humble, self-deprecating, and funny in a way that a lot of people aspire to but he achieves almost effortlessly."
Ratcliffe's decision to finish his MD training was one of the key choices that shaped his scientific career. But perhaps the biggest decision wasn't his. As a teenager growing up in northwest England, Ratcliffe wanted to become a chemist. "I got this idea I think because a relative I'd never met was said to be a very successful chemist," he says. However, his school's domineering headmaster had other ideas, pulling him out of class to advise that he become a doctor instead. "I said, 'yes, sir,'" recalls Ratcliffe, who didn't even consult his parents before making such a momentous change to his life plan. Before long, he was studying for his bachelor degree in medicine and surgery at Cambridge.
His first three years of medical training seemed to belie the headmaster's opinion. "I did badly," Ratcliffe says. "I didn't organize myself." But his difficulties gave him invaluable experience, he says. People accustomed to success can struggle in the lab because they expect all their results to be important—but there's no guarantee that they will be, he says. "Until you've failed at something, it's hard to deal with that uncertainty."
Ratcliffe turned things around when he began his clinical training at St. Bartholomew's Hospital in London. He was treating patients—something he continues to do—and enjoyed the challenge of making diagnoses. Ratcliffe liked many of the specialties he rotated through during that period, including psychiatry and oncology, but he picked nephrology. He admits that he didn't know much about the field and chose it on the basis of the recommendation of a nephrologist he respected. "He said, 'you're very good, I think you should be a nephrologist like me,'" Ratcliffe recalls. "Flattery can get you to do anything." That seemingly arbitrary choice led him to the topic that became the focus of his prize-winning research.
At that stage of his career, however, he was too busy with clinical responsibilities to tackle research projects. Instead, he began publishing case reports about patients he had treated. Scientists typically look down on case reports, Ratcliffe says, and for years he buried the ones he had written on his CV. But he now sees that preparing them sharpened his scientific thinking and "taught me how to look for potentially soluble problems amidst a mass of insoluble distractions."
After three years in London, Ratcliffe moved to Oxford in the early 1980s to finish his nephrology training. Along with his clinical work, he was spending more time on research and eventually opted to start a graduate degree in medicine. He had already conceived experiments for the degree, so after his proposed supervisor decamped, "I just stayed and carried on," he says.
Around that time, Ratcliffe saw one of those potentially soluble problems that he had learned to recognize: how cells in the body respond to changes in their oxygen supplies. The subject had a nephrology connection. The kidneys fashion most of the body's erythropoietin, the hormone that boosts the bone marrow's production of red blood cells in response to reduced oxygen availability. But researchers didn't know how oxygen scarcity prompted cellular responses in the first place.
Today, "probably thousands of individual research groups" are investigating how low oxygen levels affect the body in health and disease, says organic chemist Christopher Schofield, another of Ratcliffe's Oxford collaborators. But when Ratcliffe entered the field more than 30 years ago, "it was small and niche." Researchers usually publish more papers if they choose popular fields, Schofield says, but Ratcliffe "was unafraid to work in an unfashionable and challenging field."

Ratcliffe (back left) poses with his Oxford collaborators.
Courtesy of Peter Ratcliffe
Although Ratcliffe wanted to dig deeper into oxygen sensing, some obstacles stood in the way. For one thing, "I had no technical knowledge at all of molecular cell biology." And where he could perform experiments was unclear because his career had reached a crossroads. He wanted to stay in Oxford, where he had lived with his wife and four children for a decade. However, he also had applied for nephrology jobs at other hospitals that promised him opportunities for research. But in retrospect, he says, making progress in the lab while working as full-time nephrologist was "a truly impossible ambition." In the end, he didn't have to attempt that balancing act because he landed a position as a Wellcome Trust Senior Fellow in Clinical Science at Oxford, which enabled him to see patients but would allow ample time for research.
Over the next decade or so, Ratcliffe led a team of scientists who helped solve the oxygen-sensing mystery. Several of his qualities made the achievement possible, say researchers who collaborated with him. "He's very committed, focused, and hard-working," Schofield says. Pugh lauds Ratcliffe's gift for recognizing promising new avenues of research and directing resources into investigating them. "He's like a great football team manager," Pugh says. "If somebody [in the lab] seemed to be making a breakthrough, he'd send everything into that area."

Ratcliffe (left) chats with fellow Lasker and Nobel laureate Gregg Semenza at a 1993 conference in Woods Hole.
Courtesy of Chris Pugh
With Pugh and two colleagues, Ratcliffe made an important discovery in 1991. They identified an enhancer, a DNA sequence that revs up a nearby gene, close to the gene for erythropoietin. The enhancer, they found, increased gene activity when oxygen levels were low. Semenza's team and another group pinpointed similar sequences around the same time.
The scientists' next experiment was a watershed that upended their assumptions about oxygen sensing. The kidneys make most of the body's erythropoietin, and the liver supplies the rest. As a result, scientists at the time thought that only kidney and liver cells were attuned to oxygen. Ratcliffe and colleagues found otherwise when they inserted their enhancer into connective tissue cells, which to their surprise also seemed to have oxygen-sensing capability. Ratcliffe says that when he first saw the result, he was dismayed because it meant that the experiments he and his team had planned wouldn't work. "Then I reconnected my head and saw it had some important implications." For example, the finding suggested that the ability to sense oxygen was widespread among cells and must have functions aside from controlling erythropoietin. And when the researchers followed up on the discovery, they detected evidence for the capability "in every cell type we tested," Pugh says. Their results jibed with those from Semenza's lab.
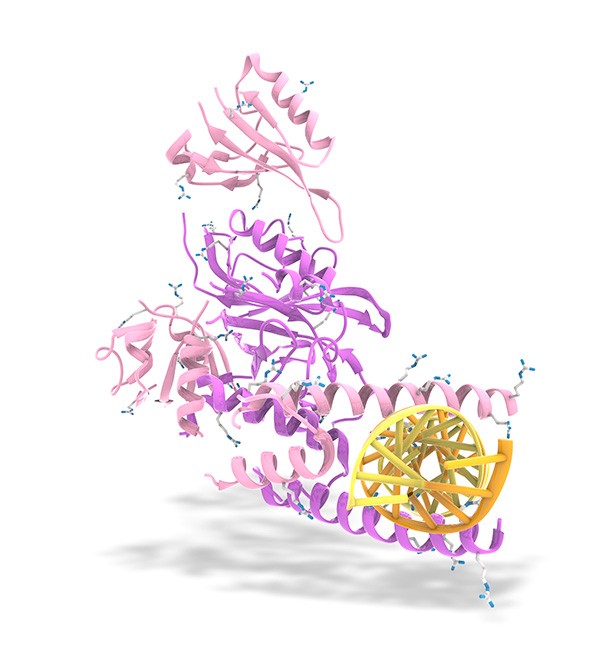
Molecular model of HIF (pink ribbon) bound to DNA (yellow double helix)
Courtesy of Ramon Andrade 3DCiencia / Science Source
More cells were sensing oxygen than expected, but how they were doing it remained murky. Semenza and a colleague found a way forward. In 1992 they identified a protein known as HIF-1 that functions as a switch, turning on cells' production of erythropoietin and other proteins when oxygen is scarce. Ratcliffe's team and other researchers found that when oxygen is plentiful, cells destroy one piece of HIF-1, known as HIF-1α, keeping the protein's levels low. In 1999, Ratcliffe and colleagues revealed that a different protein known as VHL promotes the demise of HIF-1α. More details emerged from dual 2001 papers by Ratcliffe's group and a team led by Kaelin, which showed that certain chemical modifications to HIF-1α make it more attractive to VHL. In another 2001 study, Ratcliffe joined forces with Schofield and colleagues to identify—first in nematodes and then in human cells—the enzymes that make those modifications.
The Rube Goldberg nature of the mechanism, in which cells make HIF-1α just to destroy it if oxygen is plentiful, helps explain why researchers didn't uncover it before, Ratcliffe says. "No smart person would expect it to work this way."
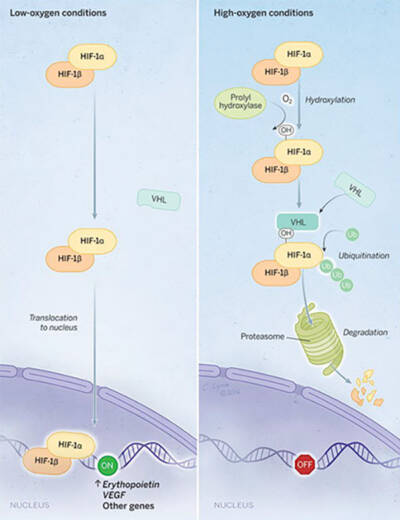
In low-oxygen conditions (left), HIF turns on production of erythropoietin and other proteins; in high-oxygen conditions (right), HIF is targeted for destruction by the proteasome.
Illustration by Cassio Lynm.
By the end of 2001, he says, the oxygen-sensing story was essentially complete, and he began to take on other responsibilities. Between 2004 and 2016, he headed the department of clinical medicine at Oxford, where he continued to work in the lab, treat nephrology patients, and practice acute medicine. After 12 years of the job, though, "I didn't want to progress up the university hierarchy of management," he says. In 2016, he took a half-time position as clinical research director at London's Francis Crick Institute. He continues to split his time between the institute and Oxford.
The discovery of the oxygen-sensing mechanism not only revolutionized researchers' understanding of a fundamental cellular function but also gave drug developers new targets. In the last five years or so, the medications known as HIF prolyl hydroxylase inhibitors, which prevent the breakdown of HIF-1α, have hit the market, aimed at patients with anemia resulting from kidney disease. Because tumor cells grow swiftly, they can run short of oxygen and often rely on HIF-1α to survive. Researchers are working to develop cancer treatments that block HIF-1α. So far, the Food and Drug Administration has approved no such therapies. But one drug that targets the related protein HIF-2α, which also helps cells cope with low oxygen levels, has been approved for treating patients with kidney cancer and for people with tumors caused by defects in the gene that encodes the VHL protein.
Ratcliffe made such an impact because "he is a consummate molecular biologist and biochemist," Johnson says. During his team's work to dissect the oxygen-sensing pathway, the researchers studied cells, genetically modified mice, fruit flies, and nematodes, Rocha notes. That diversity shows that "he is not afraid to try different approaches," she says. What's most impressive, Johnson adds, is that Ratcliffe achieved so much in science while excelling as a physician. "Not many people can do both."
By Mitchell Leslie

