In the early 1960s, cataract surgery was an ordeal that upended patients’ lives. During the operation, the surgeon made a cut that extended halfway around the eye’s front surface, reached in with forceps, and gently pulled out the cloudy lens. After the operation, patients spent up to 10 days in a hospital bed, sometimes with their head held in place by sandbags. Even when discharged, patients faced at least a month of recuperation before they could resume normal activities.
In the 1960s, Charles Kelman, an ophthalmologist at the Manhattan Eye, Ear & Throat Hospital, devised a less-invasive approach that transformed cataract surgery into an outpatient procedure. Known as phacoemulsification, the procedure requires only a small incision in the eye because the surgeon wields an ultrasonic instrument similar to those dentists use to clean patients’ teeth. The instrument shatters the lens and sucks up the fragments.
Phacoemulsification initially drew scorn from many of Kelman’s peers. “It was a huge departure” from the technique at the time, says ophthalmologist Christopher Leffler of Virginia Commonwealth University School of Medicine. But it proved more effective and easier on patients than traditional operations, and U.S. surgeons now use it to remove more than 90% of cataracts. Although researchers have devised other methods since Kelman’s day, “phacoemulsification has not been superseded,” says Leffler, who is writing a book about the history of cataract surgery.

Eye from a coffin, sculpture, Egypt, Late Period, 21st – 26th Dynasty (1081 – 525 BCE)
Courtesy of LACMA
Developing phacoemulsification earned Kelman the 2004 Albert Lasker Clinical Medical Research Award. Six other scientists and doctors also have received Lasker Awards for their efforts to improve eye health. They devised a technique for seeing deeper into the retina, helped prevent a condition that blinded and killed thousands of children every year, unlocked the first treatments for the world’s two leading causes of blindness, and channeled much-needed funds into eye research.
Try, Try Again
Kelman was a larger-than-life character who wrote a hit single, performed a lounge act in Atlantic City, and learned to fly a helicopter. He also was persistent, a quality he would need to develop a new form of eye surgery.

In a traditional cataract operation, the incision needed to be large, about 11 mm, to allow the 10mm-diameter lens to fit through. Kelman had a brainstorm, envisioning that the lens could be removed through a small incision, thereby speeding the patient’s recovery. The challenge was how to get the lens through a 2mm opening. Breaking down the lens with chemicals didn’t work, Kelman found. He then tried “several dozen instruments,” including one that a colleague described as a “gear screw–mounted, meat-grinding type of device.”
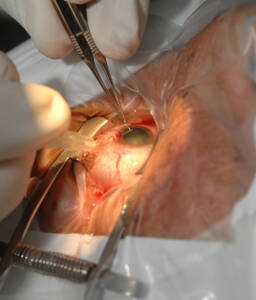
Cataract surgery
Courtesy of Medicimage/ Science Source
They were either ineffective or too destructive. Desperate, Kelman turned to an instrument he’d seen in his dentist’s office, an ultrasonic device with a rapidly vibrating tip for dislodging plaque. A similar instrument, he thought, might pulverize a cloudy lens. “The concept sounds simple, but doing it in a way that doesn’t injure the eye is a technical feat,” Leffler says. Kelman worked with the manufacturer of the dental instrument to produce a version usable for eye surgery and then tested the device on animals and more than 200 cadavers. Still, his first operation on a patient was a disaster, Kelman wrote in his autobiography.
Kelman persevered, revamping his instrument and refining the technique. By the early 1970s he was performing the operation regularly and had set up a program to teach other surgeons. Still, many of his peers remained fiercely opposed to the approach. Phacoemulsification’s popularity didn’t take off until the 1980s because the technology needed time to mature, Leffler says. By the 1990s, it was the go-to option for cataract surgery.
The Two-Cent Solution
Alfred Sommer, the 1997 winner of the Albert Lasker Clinical Medical Research Award, also had to overcome intense skepticism from other scientists to see his discoveries put into practice. Once they were, however, they saved the lives of thousands of children and spared many others from blindness. “He made a very, very important contribution,” says Clare Gilbert, an ophthalmologist and expert on international eye health at the London School of Hygiene and Tropical Medicine in the United Kingdom.
Sommer, an ophthalmologist and epidemiologist at the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, had a “holy cow experience” while poring over data from a study his team conducted in Indonesia in the late 1970s. The study had tracked about 3,500 rural children, some of whom had xerophthalmia, a group of eye conditions caused by insufficient vitamin A intake. Patients often suffer from night blindness and can permanently lose their sight. At the time, xerophthalmia was rare in rich countries such as the U.S. because most people got enough vitamin A in their diet, but it remained a problem in poorer countries.
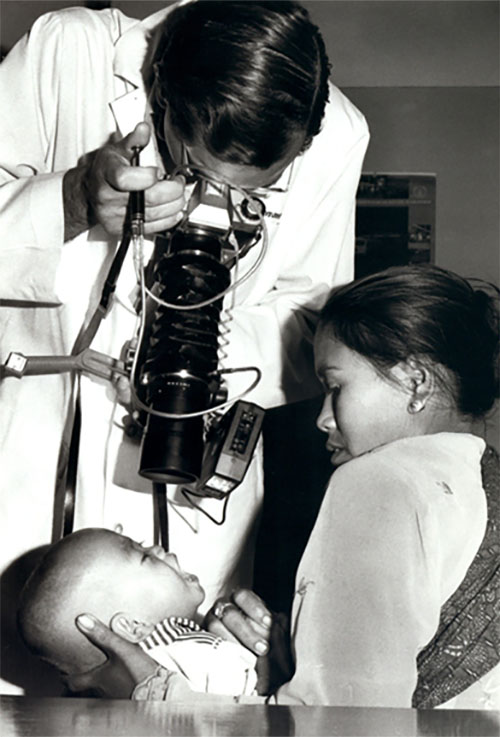
Sommer examining a young patient in Indonesia (1978)
Courtesy of Sommer
Sommer put off analyzing the data for about two years after the trial ended. One weekend in 1981, however, he started paging through the results and adding up the numbers. “Suddenly, I said, ‘what the hell is going on here?’” he recalls. He noticed that children with xerophthalmia often hadn’t come to their follow-up assessments three months later—because they had died. Even children with mild xerophthalmia were four times more likely to die than those with healthy eyes, Sommer and colleagues determined.
Sommer had discovered that vitamin A deficiency was much more severe than public health experts thought. “It was not only an important cause of blindness, it was also a source of mortality,” Gilbert says. Researchers now know that children who aren’t getting enough of the vitamin are more likely to die if they come down with infectious diseases such as measles.
At the time, however, “absolutely nobody believed it,” Sommer says. The finding implied that boosting children’s vitamin A intake could save not only their vision but also their lives. Sommer and colleagues tested that possibility in several villages in Indonesia. The mortality rate among children who received vitamin A capsules—which then cost 2 cents each—fell by about 50%.
Skeptics still weren’t convinced. However, further work by Sommer and other researchers confirmed the health payoffs of supplemental vitamin A for children deficient in the nutrient. “Over the last 30 years, blindness in children from corneal scarring, which is often associated with vitamin A deficiency, has declined dramatically in low-income countries,” Gilbert says.
Sommer says he noticed a pattern that eluded other researchers partly because “I’m sort of a right-angle thinker.” But he also had immersed himself in his data and knew it intimately. “You have to look at all the pieces of data, what drives them, and how they can be related,” he says.
A Talent for Philanthropy
Like Kelman and Sommer, 1975 Albert Lasker Public Service Award winner Jules Stein trained as an ophthalmologist. But Stein made his biggest impact on eye health through philanthropy and advocacy. In 1960, he launched Research to Prevent Blindness, a foundation that has awarded more than $413 million in scientific grants. Stein’s lobbying also was instrumental in creating the National Eye Institute (NEI), the branch of the National Institutes of Health (NIH) that supports eye research.
A musician himself, Stein practiced medicine briefly in the early 1920s before quitting to start the Music Corporation of America (later known as MCA), a talent booking agency. He and colleagues expanded and diversified the company, building it into an entertainment behemoth that represented most major Hollywood stars of the time and produced movies, TV shows, and music.

The Stein Eye Institute (UCLA) opened its doors in 1966.
Courtesy of DocFreeman24
According to his granddaughter, Stein started Research to Prevent Blindness because at the time “there wasn’t a single organization dedicated to eradicating the diseases that caused blindness.” One of the foundation’s first actions was to underwrite construction of new eye research institutes at six U.S. universities. Today it doles out about $11 million in research grants each year.

Stein also pushed to establish a separate eye institute within NIH. Despite opposition from the NIH director and other top public health officials, Stein’s efforts, including his testimony to Congress, helped get the bill creating the NEI passed in 1968. Even then, the NEI wasn’t a done deal: President Lyndon Johnson intended to veto the bill. Stein persuaded Johnson to support it instead. “For vision science and blindness prevention, [Stein] was the leading philanthropist in world history,” a fellow ophthalmologist wrote in Stein’s 1981 obituary.
No More Blood Vessels, Please
Other scientists warned Napoleone Ferrara not to start the investigation that eventually won him the 2010 Lasker~DeBakey Clinical Medical Research Award. He wanted to chase down a protein that spurs blood vessels to grow, but colleagues warned that the pursuit could be so challenging and lengthy that “it could be a career killer,” recalls Ferrara, now at the University of California, San Diego. He shrugged off their concerns because the research “was so exciting,” he recalls. “I was fascinated.”
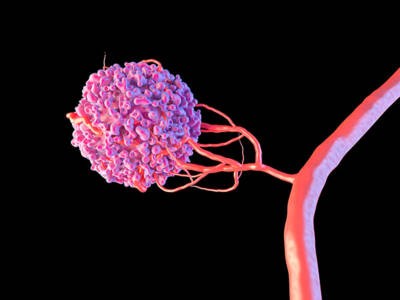
The illustration shows a malignant cell (pink) promoting the formation of new blood vessels. The cell releases angiogenic growth factor proteins, such as vascular endothelial growth factor (VEGF), that encourage the growth of new blood vessels from existing ones.
Courtsey of Maurizio de Angelis/ Science Source
Ferrara and colleagues not only nabbed the protein, known as vascular endothelial growth factor (VEGF), but also developed antibodies to stymie it. They thus delivered the first treatments for the two biggest causes of blindness worldwide: wet age-related macular degeneration (AMD) and proliferative diabetic retinopathy. For both conditions, VEGF-targeting antibodies remain the standard therapies, and they are among the most commonly administered treatments in all of medicine, notes vision scientist John Penn of Vanderbilt University School of Medicine in Nashville, Tennessee. The impact of Ferrara’s discoveries “is almost immeasurable,” he says.
The formation of new vessels, called angiogenesis, is important in cancer because tumors need a dedicated blood supply to satisfy their hunger for nutrients. Abnormal blood vessels also sprout and damage the retina in wet AMD and in proliferative diabetic retinopathy, a condition common in diabetics.
When Ferrara began the research that led to VEGF, he was studying the pituitary gland, trying to determine how it develops so many blood vessels. He identified a group of pituitary cells that might promote angiogenesis and mixed them with endothelial cells from the lining of blood vessels. “Lo and behold, they induced endothelial cell growth,” he says, a finding suggesting that pituitary cells were releasing a protein that spurs angiogenesis.
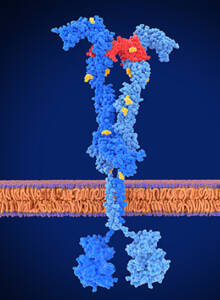
Activation by VEGF (red) leads to the dimerization of the VEGF receptor monomers.
Courtesy of Juan Gaertner / Science Source
With the technology of the time, sifting that protein from the welter of other, more abundant proteins made by pituitary cells could take years, Ferrara says. Moreover, he had to perform the work in his spare time. His day job involved investigating an experimental treatment for speeding childbirth at the biotech firm Genentech in South San Francisco, California. However, after only a couple of years, in 1989, Ferrara and his Genentech colleagues had isolated VEGF and pinpointed its gene; an independent group also identified the gene around the same time.
Once they had uncovered VEGF, Ferrara and collaborators could begin investigating how to block its effects. To curb abnormal angiogenesis, the researchers generated a monoclonal antibody that became the drug ranibizumab, which the Food and Drug Administration has approved for treating wet AMD and proliferative diabetic retinopathy. Today ophthalmologists can choose from six VEGF-thwarting antibodies. The drugs make the diseases manageable. In wet AMD, for instance, the antibodies halt formation of defective blood vessels in about 90% of patients and lead to improved vision in about 30%, Penn says. Patients are seeing more clearly, he says, because of Ferrara’s “irrepressible scientific curiosity.”
Look Deep into My Eyes
Ophthalmologists can diagnose retina diseases earlier and more easily because of the winners of the 2023 Lasker~DeBakey Clinical Medical Research Award: electrical engineer James Fujimoto of the Massachusetts Institute of Technology (MIT) in Cambridge; electronics researcher Eric Swanson, also of MIT; and David Huang, now an ophthalmologist at the Oregon Health & Science University in Portland. The trio invented optical coherence tomography (OCT), a technique that gives ophthalmologists a much more detailed view of the retina. “It enabled us to image the retina and measure disease in ways that were not possible before,” says Martin Friedlander, a retina specialist at the Scripps Clinic in La Jolla, California, who wasn’t involved in developing the approach.
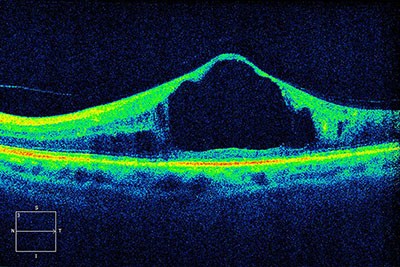
An optical coherence tomography scan of a patient’s retina shows diabetic macular edema, the most common cause of vision loss in people with diabetes.
Courtesy of Alan Frohlichstein / Science Source
Fujimoto and Huang, then an MD–PhD student in Fujimoto’s lab, began developing OCT in the 1980s. They were investigating whether a method known as interferometry could allow them to measure the thickness of the cornea and the retina. Common today in fields such as astronomy and engineering, interferometry builds on the observation that when two light beams combine, they will amplify each other if they are in phase but cancel each other if out of phase. The pair built a device that splits a beam of light, sending one of the resulting beams toward a sample and the other toward a mirror. After reflecting off their targets, the two beams recombine and proceed to a detector. By analyzing the resulting interference pattern, the researchers could determine the travel time for the light reflected from the sample and thus calculate its thickness.
When they tried the device on a sample of retina tissue, the light reflected from the retina created a complex pattern that made the thickness hard to measure, Huang recalls. “I saw that it was signal from the internal retinal layers and not noise, and recognized the opportunity to build images from it.”

From left to right: Huang, Claire Pomeroy, Fujimoto, and Swanson at the 2024 Lasker Awards Ceremony
Although their prototype could distinguish layers in the retina, the process was slow. The duo needed help from Swanson, whom Huang has described as the team’s “secret weapon.” Swanson had been developing technology for light transmission between satellites, and he designed better detectors and optical systems that greatly increased the device’s speed. In 1991, the researchers were ready to premiere OCT in a paper in Science. The team soon built an instrument for ophthalmologists to use.
At the time, the ophthalmoscope was the standard instrument for examining the eye, but it affords only a two-dimensional view. OCT yields a three-dimensional image with much higher resolution. OCT “is the ‘gold standard,’” Friedlander says.
This group of Lasker winners improved the lives of patients with eye disease, but their discoveries had a much broader impact. Ferrara and colleagues also developed a VEGF-disabling antibody that became a standard treatment for certain cancers. OCT has proven valuable not just for diagnosing eye diseases but also for a variety of other medical uses, such as guiding the insertion of stents into arteries of the heart. And phacoemulsification has led to other minimally invasive procedures common today, Leffler says. Patients who undergo laparoscopic surgery or knee ligament repair, for example, also are beneficiaries of Kelman’s vision.
By Mitchell Leslie




