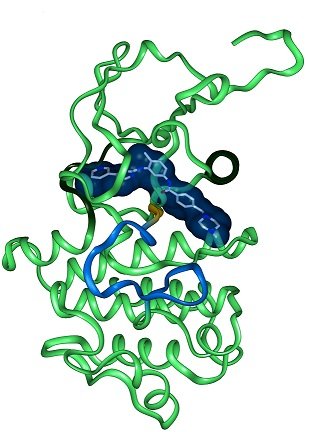
Structure of Gleevec bound to the kinase domain
Brian Druker, Nicholas Lydon, and Charles Sawyers received the 2009 Lasker~DeBakey Clinical Medical Research Award for developing targeted molecular therapy treatments for chronic myeloid leukemia (CML), a condition in which the body accumulates too many white blood cells, eventually causing death. A major part of the treatment involves a drug called Gleevec (imatinib), which was approved by the US Food and Drug Administration (FDA) in 2001.
The work of Druker, Lydon, and Sawyers converted a previously fatal cancer into a manageable chronic condition, and it provided a model for targeted molecular therapies and an example of scientific partnership between academia and industry. In this interview, the three discuss challenges related to transforming cancer research products into therapies and explore the different roles that academia and industry play in drug discovery research partnerships.
Q: How do contributions from academia and industry differ in research collaborations?
 Druker: The development of Gleevec was a shining example of an industry-academic partnership. Nick and I were true partners, sharing data, outlining workflows, and planning the clinical trials. We each did what we do well — academics identified and validated the target of Gleevec as the cause of a disease, Nick’s group developed a drug against this target, my lab demonstrated that this drug worked in disease models that we had developed, and together we planned and executed the clinical trials.
Druker: The development of Gleevec was a shining example of an industry-academic partnership. Nick and I were true partners, sharing data, outlining workflows, and planning the clinical trials. We each did what we do well — academics identified and validated the target of Gleevec as the cause of a disease, Nick’s group developed a drug against this target, my lab demonstrated that this drug worked in disease models that we had developed, and together we planned and executed the clinical trials.
 Lydon: Industry and academia scientists have different but overlapping skill sets, which are best utilized during different stages of a drug discovery and development program. Most new target research is initiated within academia. Thus, prior to starting the drug discovery program on BCR-ABL [the oncogene causing CML] at Ciba-Geigy, much groundbreaking basic research work had already been carried out within academia, which had defined the molecular pathophysiology of CML. Following the discovery of Imatinib, we established a critical translational medicine collaboration with Brian Druker to profile the use of imatinib in CML patient samples and BCR-ABL-transformed cell lines. This translational work was critical in reducing the project’s risk prior to clinical testing and allowed an investment argument to be made to the company for the development of imatinib for CML. This was not easy, as CML was perceived as an orphan indication, and there was significant competition for development funding within the company. Following preclinical development, both Brian and Charles Sawyers then collaborated on the clinical testing of this novel targeted therapy in CML patients. This collaboration with physician-scientists, who had deep understanding of CML, both during the development of the clinical plan and then during the clinical trials, was critical for the rapid clinical proof of concept and FDA approval of imatinib. This model still holds today.
Lydon: Industry and academia scientists have different but overlapping skill sets, which are best utilized during different stages of a drug discovery and development program. Most new target research is initiated within academia. Thus, prior to starting the drug discovery program on BCR-ABL [the oncogene causing CML] at Ciba-Geigy, much groundbreaking basic research work had already been carried out within academia, which had defined the molecular pathophysiology of CML. Following the discovery of Imatinib, we established a critical translational medicine collaboration with Brian Druker to profile the use of imatinib in CML patient samples and BCR-ABL-transformed cell lines. This translational work was critical in reducing the project’s risk prior to clinical testing and allowed an investment argument to be made to the company for the development of imatinib for CML. This was not easy, as CML was perceived as an orphan indication, and there was significant competition for development funding within the company. Following preclinical development, both Brian and Charles Sawyers then collaborated on the clinical testing of this novel targeted therapy in CML patients. This collaboration with physician-scientists, who had deep understanding of CML, both during the development of the clinical plan and then during the clinical trials, was critical for the rapid clinical proof of concept and FDA approval of imatinib. This model still holds today.
 Sawyers: I think academic research is really critical for laying the foundation of basic understanding of cancer and pointing towards certain targets or drug possibilities. BCR-ABL was discovered by the academic community, but Gleevec was discovered by the pharmaceutical community. Pharmaceutical companies are unbelievably good at finding a drug once they know what to look for, optimizing it, testing it in humans safely, and conducting clinical trials. And when a drug starts to work, they can quickly move into large scale and roll it out internationally. The academic community comes in [again] a bit later and is really critical in the actual clinical development of the drug because sometimes the target is identified, the drug is made, it’s tested in some patients, and it doesn’t exactly play out the way the laboratory science suggests. And there you need astute academic clinicians to figure out — all right, you thought it was going to do this but actually it does that, and here’s a new way that we can identify the patients that are most likely to benefit.
Sawyers: I think academic research is really critical for laying the foundation of basic understanding of cancer and pointing towards certain targets or drug possibilities. BCR-ABL was discovered by the academic community, but Gleevec was discovered by the pharmaceutical community. Pharmaceutical companies are unbelievably good at finding a drug once they know what to look for, optimizing it, testing it in humans safely, and conducting clinical trials. And when a drug starts to work, they can quickly move into large scale and roll it out internationally. The academic community comes in [again] a bit later and is really critical in the actual clinical development of the drug because sometimes the target is identified, the drug is made, it’s tested in some patients, and it doesn’t exactly play out the way the laboratory science suggests. And there you need astute academic clinicians to figure out — all right, you thought it was going to do this but actually it does that, and here’s a new way that we can identify the patients that are most likely to benefit.
Q: Now that new knowledge of cancer biology has made it possible to make cancer drugs, will this provide an incentive to industry to shoulder some of the basic research funding to make up for reduced public funding?
Lydon: I believe industry can make up for some of the public funding shortfall by leveraging academic relationships to conduct early-discovery and biology studies to support drug discovery and translational studies. Currently, industry spends a significant amount of money on sponsored research in academia. However, this type of funding is not for everyone, as it is primarily focused on answering specific disease-related questions rather than generating basic research knowledge. For companies, funding more basic research would not be wise, given that the primary function of the pharmaceutical industry is drug discovery and development.
Sawyers: I don’t think pharmaceutical companies are in a position to convince their shareholders to pay for basic research. I don’t think that’s going to happen. They’re going to spend a lot of research dollars — which they do — but it’s going to be on much more applied science, the very expensive research that’s required to take an academic finding and make it into a drug.
Druker: It is absolutely clear that an enormous amount of public funding went into academic laboratories that identified and validated the target of Gleevec. Industry already spends quite a bit on research and development, and they fund projects in many academic laboratories that support the products they are developing. As much as I would like for industry to contribute more to general research funding, for many reasons I can’t imagine this happening. With this in mind, we need to continue to advocate for increased public funding and private philanthropy to support basic research.
Q: How can cancer research advance more quickly from the lab to therapies?
Druker: Difficult question in that what is needed is more knowledge. For example, if you told me I could have access to all of the cancer data in the world right now, I don’t actually think I would be able to make a huge impact on patient outcomes. This is because we still don’t know why many therapies work or why they fail. We also don’t have great models for how best to combine therapies or predict toxicities. But, we can prepare for the deluge of information that will be coming as sequencing of patients’ tumors becomes more commonplace. By linking [sequencing data] to treatments and outcomes, we will get a much clearer view of why treatments work, don’t work, or stop working. Establishing the infrastructure for data sharing will be critically important to accelerate progress.
 Sawyers: It’s important that basic research continues to be funded, because if you trace the origins of a lot of these targeted therapies, it was not a drug company thinking “let’s make a treatment against this disease.” The success of Gleevec combined with all the groundwork that was laid by the cancer biology field led to a very ripe moment in time. There’s a lot of good targets now that if we had drugs against them would lead to benefits for patients, but we don’t know how to make drugs against them. These are the so-called ‘undruggable’ targets. That’s a very large unmet need for the field, and whether or not the pharmaceutical industry can do that s an open question.
Sawyers: It’s important that basic research continues to be funded, because if you trace the origins of a lot of these targeted therapies, it was not a drug company thinking “let’s make a treatment against this disease.” The success of Gleevec combined with all the groundwork that was laid by the cancer biology field led to a very ripe moment in time. There’s a lot of good targets now that if we had drugs against them would lead to benefits for patients, but we don’t know how to make drugs against them. These are the so-called ‘undruggable’ targets. That’s a very large unmet need for the field, and whether or not the pharmaceutical industry can do that s an open question.
Lydon: A clear and robust understanding of target biology is a critical factor in the success of any drug dicovery project. Much of the early insights into target biology are discovered within academia. However, it is often difficult for industry to collaborate effectively with public institution at the drug discover stage, as they have very different missions and expectations. Additionally, many potential collaborations are thwarted by questions regarding the ownership of intellectual property and the academic need for early publication. An area with fewer issues is translational medicine, which is increasingly important to drug development. Collaborations with physician-scientists in areas such as early ‘clinic proof of concept’ and biomarker development are important way to reduce clinical development risk and cost.
Q: What kind of support from society does cancer research need?
Sawyers: I think that each of us should decide if basic research and science that can help impact our quality of life, and particularly health, is a fundamental right and a public good. I think that it is and a lot of other people think it is too, and therefore we should be happy to contribute to that some portion of our tax dollars. That’s one type of support. The other is — with all of this genomic data that is being measured on patients in a lot of places now in real time in the clinic, patients should be willing to share their data. We could wait for institutions to work out all the barriers to sharing data, but patients have the right to own their data, and, if they’re willing, they should donate their data to public databases. I certainly would — if I had cancer and had my tumor sequenced, I would definitely donate my data.
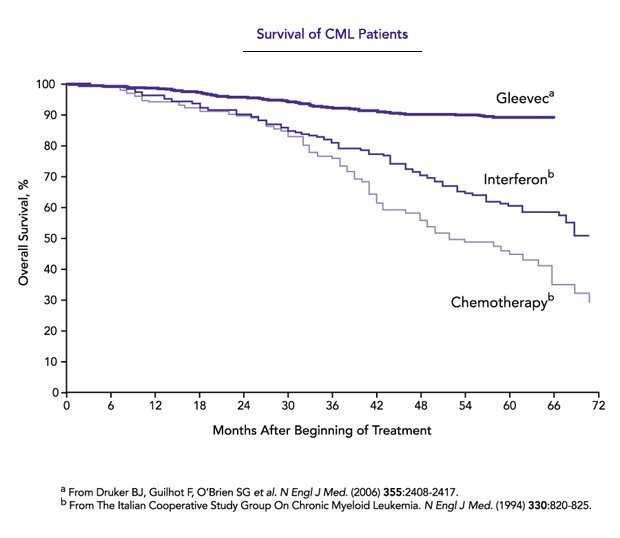
Druker: This is actually a two-way street. Yes, we would like more funding, but there are many competing priorities — education, student debt, access to health care, and many others. There are compelling economic and societal arguments for why research should be supported. As publicly funded scientists, we must strive to effectively communicate our work and its importance to the public. To do this successfully, we have to improve our abilities to speak in a manner that is interesting and approachable by the public.
Lydon: Negative press relating to drug pricing has eroded public trust in the pharmaceutical industry. Examples include annual pricing increases without any inflationary logic or rationale. The business models of a small number of ‘specialty pharma’ companies, that have effected extraordinary price increases without offering any additional value to patients, has tainted the public perception of the entire sector. Although such practices reflect a minority of the biotech/pharma industry, each such instance perpetuates a further negative stereotype of the industry as a whole. We all need to spend more time speaking to the value that we offer to patients, particularly in the transformational options that the industry has developed for diseases that were otherwise untreatable. As an industry, we need to focus on innovation and developing meaningful new treatments for patients.

Charles Sawyers, Brian Druker, and Nicholas Lydon at the 2009 Lasker Awards Ceremony
Brian Druker is the director of the Oregon Health and Science University Knight Cancer Institute, JELD-WEN Chair of Leukemia Research, and an Investigator at Howard Hughes Medical Institute.
Charles Sawyers is an investigator of the Howard Hughes Medical Institute and chair of the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Center.
Nicholas Lydon is founder and BOD member of BluePrint Medicines, AnaptysBio, Staurus Pharma LLC. Previously, he was at Ciba-Geigy, which later became Novartis.
The interviews above have been edited for length and clarity. The interview with Charles Sawyers was conducted over the phone; Brian Druker and Nicholas Lydon answered questions over email.
