In November 1967, 26-year-old Joan Steitz walked into the MRC Laboratory of Molecular Biology (LMB) in Cambridge, UK. Fresh off completing a molecular biology PhD at Harvard, Steitz eagerly awaited a postdoctoral research position at the LMB. But the facility’s director had dropped the ball. The LMB, one of the world’s foremost institutions for studying molecular biology, had for her neither lab space nor a project. The director suggested she try a library project instead, but she wasn’t about to spend the next three years stuck in the stacks. “I knew that experimental work, not theoretical work, was my forte,” she recalled in a 2022 interview.

Steitz presents at the Cold Spring Harbor Symposium on Quantitative Biology Structures of DNA, 1982.
Courtesy of Cold Spring Harbor Laboratory Archives
Steitz’s response to that setback showed qualities that made her one of the most successful and respected scientists in her field. “She is dogged,” says Yale biochemist Susan Baserga, who was a postdoctoral researcher in Steitz’s lab in the late 1980s and early 1990s. To find her own project, Steitz went from lab to lab at the LMB, canvassing other researchers and postdocs for their best ideas. She also persuaded LMB staff member Mark Bretscher to give her some workspace, a three-foot-long section of a lab bench barely big enough to run experiments.
Another of her characteristics is fearlessness, says RNA biologist Sandra Wolin of the National Cancer Institute in Bethesda, Maryland, who was Steitz’s graduate student at Yale. That attribute showed in the project Steitz chose, a study the other postdocs—all men—had shied away from because it seemed too hard. The work involved probing the interaction between messenger RNA (mRNA), which carries the instructions for making specific proteins, and ribosomes, the RNA-containing organelles that read those instructions and build proteins. Steitz determined how ribosomes recognize mRNAs, publishing her findings in 1969 in Nature.
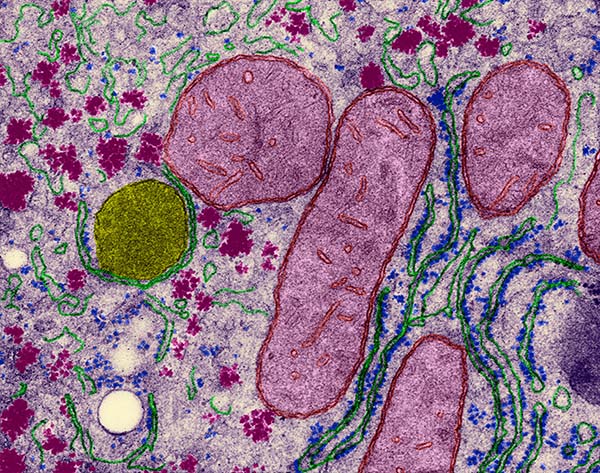
Cellular organelles from a hepatocyte – mitochondria (light purple); endoplasmic reticulum (green); ribosomes (blue); glycogen granules (dark purple); peroxisome (yellow); lipid bodies (white), colored transmission electron micrograph. Magnification: x11,440 when shortest axis printed at 25 millimeters.
Courtesy of Dennis Kunkel Microscopy / Science Source
That result set the stage for a career that helped transform how researchers think about RNA. When Steitz started in science, most researchers saw the molecule as DNA’s less-interesting cousin, responsible only for the menial jobs necessary to produce proteins. Thanks partly to Steitz’s findings, scientists now know RNA is versatile and carries out a range of vital tasks: managing cells’ protein repertoires, serving as an enzyme, fighting viruses, and even editing itself. “I would put her in the top four” most influential scientists in RNA research, says Harvard molecular biologist Frank Slack.
A standing ovation for Steitz at the 2018 Lasker Awards Ceremony
Steitz later received the 2018 Lasker~Koshland Special Achievement Award in Medical Science for her discoveries, expanding the field of RNA research, and nurturing the careers of the next generations of scientists. “Her impact is huge,” says George Calin, an RNA researcher at the University of Texas MD Anderson Cancer Center in Houston. “She had the dedication to grow a field that seemed initially to be of secondary importance.” Her influence has been particularly important for women, who were almost unknown in molecular biology when Steitz entered the field. “It was Joan’s belief in my work that gave me the confidence to move forward,” Baserga says.
Steitz was born in Minneapolis, Minnesota, in 1941 and grew up there. Her interest in science blossomed early with rock and butterfly collecting. The small girls’ high school she attended offered only three science classes, and Steitz took them all. Her mother, a speech therapist, and her father, a high school guidance counselor, encouraged her scientific pursuits and urged her to aim for a career and “not just become an incubator” for children, she says.
As an undergraduate at Antioch College in Ohio, Steitz majored in chemistry. However, the school’s work program, in which students sampled jobs around the country and overseas, made a bigger impact on her life than the coursework. “They send you off into the world on a quarter-by-quarter basis,” Steitz says. In one stint, she served as a technician in the MIT lab run by pioneering molecular biologist Alexander Rich, where she investigated whether heated ribosomes could bounce back to their original structure when they cooled down. The work was routine. She spent three months heating and cooling solutions of ribosomes and measuring how much light they absorbed. Although the experiment “turned out to be a total failure,” she says the experience sparked a lifelong fascination with RNA. “I got hooked on RNA, and since then everything I have done has to do with RNA,” she says.
Still, almost no women were running labs then, and she couldn’t imagine having a career as an independent scientist. The few women who got PhDs typically worked as research associates, or junior scientists, in labs headed by men. Becoming a doctor was a more likely but still science-related option for a woman, so she applied to medical school at Harvard, which accepted her.
Steitz never made it to medical school because of one of the serendipitous events she says have shaped her life. Because Steitz hadn’t seen her parents in four years, she decided to spend the summer at home in Minneapolis before enrolling at Harvard. To gain more scientific experience, she applied for temporary jobs in several labs at the University of Minnesota. Only cell biologist Joseph Gall, who later won the 2006 Albert Lasker Award for Special Achievement in Medical Science, agreed to hire her. Unlike at MIT, where she did what other researchers asked, Steitz could pursue her own project in Gall’s lab, inspiring her to change her plans. “By August 1, I had decided I wanted to make discoveries in science,” Steitz says.

James Watson’s and Walter Gilbert’s students and postdocs on the Harvard University Biology Department rhino, 1965
Courtesy of Cold Spring Harbor Laboratory Archives
That decision meant switching to Harvard’s biochemistry and molecular biology program—and fighting some ingrained stereotypes. When she asked a well-known professor about graduate student opportunities, he harrumphed, “But you’re a woman. What will you do when you get married and have a family?” She had better luck with molecular biologist James Watson, who shared the 1960 Albert Lasker Basic Medical Research Award and the 1962 Nobel Prize in physiology or medicine for helping discover DNA’s structure. He agreed to take her on as a graduate student. She was the first woman graduate student in his lab and the only one in her entering class of 10 students.
DNA and proteins still dominated molecular biology research then, but scientists were starting to pay more attention to RNA. For her PhD project, Steitz studied a bacteria-attacking virus whose genome was made of RNA instead of DNA. Her work involved understanding how an individual virus formed from its molecular building blocks.

Steitz and her husband Thomas Steitz at Harvard, 1967
Courtesy of Joan Steitz
The sexist professor who had rebuffed her inquiries proved to be right about one thing—she did get married while at Harvard. Her husband, Tom Steitz, was a PhD student studying protein crystallography. The pair put off starting a family for more than a decade so they could focus on their careers. When they finished their PhDs in 1967, they faced a tricky decision: where to move for their postdoctoral research. In her husband’s field, the LMB was the world’s best, so they opted to go there. Steitz recalls that Watson wrote a letter to the lab’s director that recommended her because “she’s intelligent and nice to have around.” Even at that stage, however, Steitz did not expect to eventually look for a faculty job. That attitude gave her the freedom to pick a hard project that might not pan out. By contrast, her male peers focused on beefing up their résumés before they applied for university positions, so they had to choose projects likely to succeed, she says.

“Lifelong friendships were forged at LMB,” says Steitz, here in the Swiss Alps in 1970s, looking at a map, her husband standing, Jerry Adams and Suzanne Cory sitting, two scientists now at the Walter and Eliza Hall Institute of Medical Research, Australia.
Courtesy of Mark Bretscher
The question Steitz tackled was how a ribosome “chooses” where to latch onto an mRNA molecule. Working at her three feet of lab bench space, Steitz devised a procedure that involved mixing ribosomes with mRNAs from the same virus she had studied at Harvard. When the ribosomes attached to RNA molecules, she added an enzyme that sliced up any exposed RNA strands. The stretch of mRNA grabbed by the ribosome escaped destruction because it was tucked away inside the organelle. By isolating those sheltered fragments and then sequencing them by using a technique developed by another LMB researcher, she showed that ribosomes home in on particular sequences of nucleotide bases in mRNA molecules.
Steitz’s results caught molecular biologists’ attention because she was the first to pinpoint any of those ribosome-attracting sequences. Despite that achievement, Steitz wasn’t sure she could land a job running her own lab. Although academic opportunities for women were opening up, some universities hung on to old prejudices. When her husband interviewed at the University of California, Berkeley, for example, he asked whether the school could offer her a job. The department chairman replied, “Our wives are research associates in labs, and they are so happy at that,” Steitz recalls. She and her husband instead opted for Yale, which offered both of them faculty positions.

Thomas Steitz and Joan Steitz at the Cold Spring Harbor Symposium on Quantitative Biology DNA Replication and Recombination, 1978
Courtesy of Cold Spring Harbor Laboratory Archives
They thrived at Yale and stayed there for the rest of their academic careers. Tom’s research earned him the 2009 Nobel Prize in chemistry. Joan started a lab that trained many top RNA researchers. Scientists who learned there extol her scientific acumen, mentoring style, and concern for students and postdocs. Steitz combined research rigor with humanity, says Manny Ares, a molecular biologist at the University of California, Santa Cruz. Although Ares was a postdoc in a different lab, he often sought Steitz’s advice. “Joan is steely cold about scientific ideas but warm and understanding about the human aspects of doing science,” he says. Molecular biologist Stephen Mount of the University of Maryland in College Park, who was her graduate student, is equally effusive: “She’s the reason I am the scientist I am today.” Mount says she set clear expectations for her graduate students and postdocs but allowed them to devise their own solutions, helping them grow as scientists. And people in the lab always knew how well they were performing from her expressions, he says. “She has a radiant smile, and she has this very disturbing scowl.”

Richard Axel, Steitz and Lee Hood at the Cold Spring Harbor Symposium on Quantitative Biology Structures of DNA, 1982
Courtesy of Cold Spring Harbor Laboratory Archives
Steitz has earned plaudits for her success at mentoring female researchers and for serving as a role model, but she didn’t consciously decide to step up for women in science. “I think you can’t help but be an advocate for women in science if you are a woman scientist,” she told an interviewer in 2018. Her mentoring approach works so well because it “doesn’t require doing anything extraordinary,” Wolin says. “She just gives women scientists the same support and encouragement as the men.”

Phillip Sharp and Steitz at Cold Spring Harbor Laboratory’s History of Science Meeting on mRNA Splicing, 2017
Courtesy of Cold Spring Harbor Laboratory Archives
“Some scientists live on one discovery,” but Steitz has delivered new results “with almost metronomic frequency,” Calin says. Her most significant finding helped reveal a surprising role for RNA. In the late 1970s, researchers including MIT’s Phillip Sharp, who won the 1988 Albert Lasker Basic Medical Research Award, discovered that eukaryotes—organisms whose cells carry nuclei—add a twist to protein synthesis. Eukaryotic cells produce rough drafts of mRNAs that contain extraneous sections, known as introns, that don’t code for proteins. Before using those RNAs to direct protein synthesis, cells use a process called RNA splicing to snip out the introns and rejoin the remaining fragments.
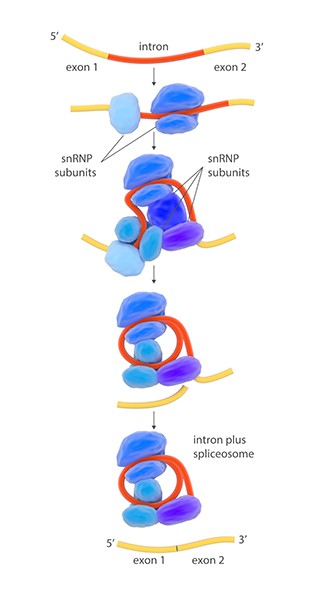
Illustration of the splicing mechanism in a eukaryotic cell. The segment of RNA to be removed, the intron, is shown in red. An enzyme complex called a spliceosome excises the intron and merges the two sections (exons) to form the mature messenger RNA (mRNA). The spliceosome consists of small nuclear ribonucleoprotein particle (snRNP) subunits (blue and purple).
Courtesy of SPL / Science Source
The mystery was how cells pare away introns. Steitz and her team suspected that mysterious protein-and-RNA-containing particles called small nuclear ribonucleoproteins (snRNPs; pronounced “snurps”) did the job. But the researchers struggled to isolate those particles until they got another of those lucky breaks Steitz says have been so important for her career. Patients with certain autoimmune diseases such as lupus make antibodies that attack the cell nucleus, and the scientists thought they could use those antibodies to fish out snRNPs—if they could get their hands on some of those proteins. Then one day, a new MD–PhD student in the lab, Michael Lerner, said he knew a doctor in the medical school who might have spare blood samples from lupus patients. Later that day, Lerner returned to the lab with some of those samples. In 1979 the researchers showed they could use the antibodies to isolate snRNPs, a key step in understanding the particles’ function. Over the next few years, Steitz and her lab members, including Wolin and Mount, along with scientists at other institutions, confirmed that snRNPs were essential for RNA splicing.

Steitz in her lab, 2018
Over the next decades, Steitz continued to investigate snRNPs and other types of RNA, including snoRNPs (small nucleolar ribonucleoproteins), which help cells make ribosomes, and microRNAs, which allow cells to fine-tune their protein output. She turned 83 this year and has entered what she calls a phased retirement, though she continues to do some scientific work. Steitz made such a significant impact, Ares says, because of her scientific imagination. She doesn’t just see the immediate implications of a result; “it’s the over-the-horizon stuff.

Steitz at Yale, 2018
She has that special vision.” Maybe equally important was her ability to persevere through many difficulties, which stems from her “unbounded optimism,” Baserga says. “Nothing ever gets her down. It’s such a great way to live.”
By Mitchell Leslie

